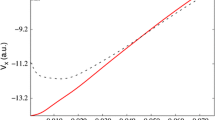
Abstract
Ab initio MO-SCF wave functions are derived for H2S for different bond angles by using a double-zeta type set of gaussian s and p orbitals. The predicted equilibrium bond angle is 95.5°. The computed value of the total electronic energy is expected to be near the Hartree-Fock limit for the molecule. The predicted value of the dipole moment does not show significant improvement with respect to similar computations not including polarisation functions.
Zusammenfassung
Ab inittio-Funktionen aus Doppel-ζ-Gaussfunktionen werden für H2S bei verschiedenen Valenzwinkeln angegeben. Die tiefste Energie (95.5°) dürfte nahe beim Hartree-Fock-Wert liegen. Die Einführung von polarisierenden Funktionen liefert hinsichtlich des Dipolmomentes keine Verbesserung.
Résumé
Des calculs non empiriques pour plusieurs valeurs de l'angle de liaison ont été effectués pour H2S avec une base étendue de fonctions gaussiennes s et p. L'angle d'équilibre prévu est de 95.5°. L'énergie électronique totale est estimée très proche de la limite Hartree-Fock pour la molécule. La valeur du moment dipolaire calculé est pareille à celle qui a été trouvée par d'autres calculs n'incluant pas des fonctions de polarisation.
Similar content being viewed by others
References
Rauk, A., Csizmadia, I. G.: Canad. J. Chem. 46, 1205 (1968).
—, Wolfe, S., Csizmadia, I. G.: Canad. J. Chem. 47, 113 (1969).
Hillier, I. H., Saunders, V. R.: Chem. Physics Letters 4, 163 (1969).
Moccia, R.: J. chem. Physics 40, 2186 (1964).
Banyard, K. E., Hake, R. B.: J. chem. Physics 41, 3221 (1965).
Boer, F. P., Lipscomb, W. N.: J. chem. Physics 50, 989 (1969).
Polezzo, S., Stabilini, M. P., Simonetta, M.: Molecular Physics 17, 609 (1969).
Clementi, E., Davis, D. R.: J. comput. Physics 2, 223 (1967).
David, D-J.: IBMOL CDC 6600 Version. Technical Report of C. C. ENSJF and Lab. de Chimie ENS, Paris 1969.
Veillard, A.: Theoret. chim. Acta. (Berl.) 12, 405 (1968).
Huzinaga, S.: J. chem. Physics 42, 1293 (1965).
Sutton, L. E.: Tables of interatomic distances. London: The Chemical Society 1958.
Clementi, E.: J. chem. Physics 40, 1944 (1964).
Cottrell, T. L.: The strengths of chemical bonds. London: Butterworths 1968.
Cade, P. E., Huo, W. M.: J. chem. Physics 47, 649 (1967).
Siegbahn, K., Nordling, C., Johansson, G., Hedman, J., Heden, P. F., Hamrin, K., Gelius, V., Bergmark, T., Werme, L. O., Manne, R., Baer, Y.: ESCA applied to free molecules. Amsterdam: North Holland 1969.
Palmieri, P., Zauli, C.: Theoret. chim. Acta (Berl.) 7, 89 (1967).
Carroll, D. G., Armstrong, A. T., McGlynn, S. P.: J. chem. Physics 44, 1865 (1966).
McClellan, A. L.: Tables of experimental dipole moments. San Francisco: Freeman 1963.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cadioli, B., Pincelli, U., Bendazzoli, G.L. et al. Ab initio computations on H2S: LCAOSCF wave functions without d orbitals. Theoret. Chim. Acta 19, 66–70 (1970). https://doi.org/10.1007/BF00527378
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00527378