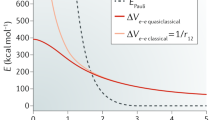
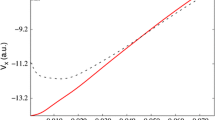
Abstract
The semi-empirical extended Hückel molecular orbital (EHMO) and non-empirical self consistent field molecular orbital (SCF-MO) methods have been used to study protonation reactions of acids, amides and esters. The work has been extended to the charged intermediates and subsequent products in their “hydrolysis” reactions.
Zusammenfassung
Ein semiempirisches, erweitertes Hückel-Verfahren sowie ab initio SCF-MO-Rechnungen wurden zur Untersuchung der Protonierung von Säuren, Amiden und Estern, deren ladungstragender Ab-kömmlinge und ihrer Folgeprodukte herangezogen.
Résumé
La méthode de Hückel étendue semi-empirique (EHMO) et la méthode du champ selfconsistant non empirique (SCF MO) ont été utilisées pour l'étude des réactions de protonation des acides, des amides et des esters. Ce travail a été étendu aux intermédiaires chargés et aux produits résultants lors de leur «hydrolyse».
Similar content being viewed by others
References
Herndon, W. C., and L. H. Hall: Theoret. chim. Acta (Berl.) 7, 4 (1967).
Rappe, C: Acta chem. scand. 20, 2236 (1966).
Ingold, C. K.: Structure and mechanism in organic chemistry, p. 754. Ithaca: Cornell University Press 1953.
Hoffmann, R.: J. chem. Physics 40, 2480 (1964).
—: J. chem. Physics 39, 1397 (1963).
Coulson, C. A.: Valence, p. 41. Oxford University Press 1952.
Pritchard, H. O., and H. A. Skinner: Chem. Reviews 55, 745 (1955).
Wolfsberg, M., and L. Helmholtz: J. chem. Physics 20, 837 (1952).
Robb, M. A., and I. G. Csizmadia: Theoret. chim. Acta (Berl.) 10, 269 (1968).
Csizmadia, I. G., M. C. Harrison, J. W. Moskowitz, S. S. Slung, B. T. Sutcliffe, and M. P. Barnett: The Polyatom System, Technical Notes No. s 36 and 40, Co-operative Computing Laboratory, M.I.T. (unpublished) Submitted to the Quantum Chemistry Program Exchange, Catalogue No. 47A.
“Tables of Interatomic Distances"” Chem. Soc. (London), Special Publications No. 11 (1958) and No. 18 (1965).
Gillespie, R. J., and T. Birchall: Canad. J. Chem. 41, 148 (1963).
Hogeveen, H., A. F. Bickel, C. W. Hilbers, E. L. Mackor, and C. MacLean: Chem. Comm. 1966, 878.
Winstein, S., M. Brookhard, and G. C. Levy: J. Amer. chem. Soc. 89, 1735 (1967).
Hogeveen, H., A. F. Bickel, C. W. Hilbers, E. L. Mackor, and C. MacLean: Rec. Trav. Chim. 86, 687 (1967).
Stewart, R., and K. Yates: J. Amer. chem. Soc. 82, 4059 (1960).
Maciel, G. E., and D. D. Trafficante: J. physic. Chem. 69, 1030 (1965).
Duffy, J. A., and J. A. Leisten: J. chem. Soc. (London) 1960, 545.
Hopkinson, A. C., N. K. Holbrook, K. Yates, and I. G. Csizmadia: J. chem. Physics 49, 3596 (1968).
Rein, R., N. Fukuda, H. Win, G. A. Clarke, F. E. Harris: J. chem. Physics 45, 4743 (1966).
Pritchard, H., and A. G. Harrison: J. chem. Physics 48, 2827 (1968).
Clementi, E.: J. chem. Physics 46, 3851 (1967).
Ritchie, C. D., and H. F. King: J. Amer. chem. Soc. 88, 1069 (1966).
Bender, M. L., and R. D. Ginger: J. Amer. chem. Soc. 77, 348 (1955).
Lane, C. A.: J. Amer. chem. Soc. 86, 2521 (1964).
Bell, R. P.: The proton in chemistry, p. 24. Ithaca: Cornell University Press 1959.
Author information
Authors and Affiliations
Additional information
Contribution from the Department of Chemistry, University of Toronto, Toronto 5, Ontario, Canada.
Rights and permissions
About this article
Cite this article
Hopkinson, A.C., McClelland, R.A., Yates, K. et al. An attempted application of the extended hückel molecular orbital approach to reactions involving charged species. Theoret. Chim. Acta 13, 65–78 (1969). https://doi.org/10.1007/BF00527320
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00527320