Abstract
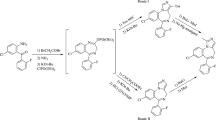
A Β-elimination reaction with the formation of 2-imino-3-vinylbenzimidazolines occurs simultaneously with intramolecular alkylation and the formation of an imidazoline ring in the action of alcoholic alkali on 2-imino-3-(2-chloroethyl) benzimidazolines. The thermolysis of 3-chlorethyl-substituted imines without a solvent or in an inert solvent leads exclusively to 2,3-dihydroimidazo[1,2-a] benzimidazoles. An attempt to obtain the latter directly from 2-imino-3-(2-hydroxyethyl) benzimidazolines by the action of a mixture of thionyl chloride and acetic anhydride on them also leads to ambiguous results.
Similar content being viewed by others
Literature Cited
V. A. Anisimova, T. B. Korochina, N. I. Avdyunina, and A. M. Simonova, Khim. Geterotsikl. Soedin., No. 3, 339 (1986).
W. L. Mosby, “Heterocyclic systems with Bridgehead nitrogen atoms,” in: The Chemistry of Heterocyclic Compounds, Interscience, New York (1961).
A. Weissberger and E. C. Taylor (editors), Special Topics in Heterocyclic Chemistry, Interscience, New York-London (1977).
A. M. Simonov and P. M. Kochergin, Khim. Geterotsikl. Soedin., No. 2, 316 (1965).
P. M. Kochergin, Khim. Geterotsikl. Soedin., Collective Vol. 1 (1967), p. 137.
R. I. North and A. R. Day, J. Heterocycl. Chem., 6, No. 5, 655 (1969).
A. C. White and R. M. Black, British Patent No. 1476949; Ref. Zh. Khim., 60157P (1977).
V. P. Kruglenko and M. V. Povstyanoi, Khim.-farm. Zh., No. 7, 61 (1979).
V. A. Anisimova, M. V. Levchenko, and A. F. Pozharskii, USSR Inventor's Certificate No. 952848; Byull. Izobr., No. 31, 127 (1982).
Chimetron S. a. r. l., French Patent No. 1488268; Chem. Abstr., 69, 59243 (1968).
Chimetron S. a. r. l., French Patent No. 1488270; Chem. Abstr., 69, 59248 (1968).
Yu. M. Yutilov, V. A. Anisimova, and A. M. Simonov, Khim. Geterotsikl. Soedin., No. 3 416 (1965).
V. A. Anisimova, A. M. Simonov, and T. A. Borisova, Khim. Geterotsikl. Soedin., No. 6, 791 (1973).
Author information
Authors and Affiliations
Additional information
See [1] for communication 21.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 7, pp. 918–925, July, 1986.
Rights and permissions
About this article
Cite this article
Anisimova, V.A., Levchenko, M.V. & Pozharskii, A.F. Research on imidazo[1,2-a]benzimidazole derivatives. 22. Synthesis of 2,3-dihydroimidazo[1,2-a]benzimidazoles starting from 2-imino-3-(2-hydroxyethyl)benzimidazolines. Chem Heterocycl Compd 22, 732–739 (1986). https://doi.org/10.1007/BF00522731
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00522731