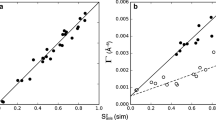
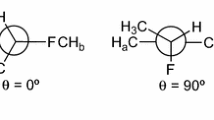
Abstract
It is proposed that a distinction should be made between stereospecificity of the first kind, which provides spectral (line assignments in the NMR spectrum) and structural (molecular conformations) information, and stereospecificity of the second kind, which only provides spectral information, on the basis of the relaxation times T1. The nature of the stereospecificity was revealed in detail during analysis of the reasons for the differences in the proton relaxation times of enantiotopic gem-dimethyl groups in the conformationally rigid molecule of 2-oxo-5,5-dimethyl-1,3,2-dioxathiane. It is suggested that the barrier to rotation of the methyl in the geminal CH3-C-H fragment can be assessed from the temperature dependence of the t1 time of the methine proton. The experiment was carried out with the stereoisomeric r-2-tert-butyl-4-methyl-trans-7-methyl[2]1,3-dioxepane.
Similar content being viewed by others
Literature cited
L. G. Werbelow and D. M. Grant, “Intramolecular dipolar relaxation in multispin systems,” Adv. Magn. Reson., 9, 189–299 (1977).
L. D. Hall, “Spin-lattice relaxation: A fourth dimension for proton NMR spectroscopy,” Chem. Soc. Revs., 14, No. 3, 401–420 (1975).
L. D. Hall and C. M. Preston, “A configuration dependence of the longitudinal relaxation times of carbohydrate derivatives,” J. Chem. Soc. Chem. Commun., No. 24, 1319, (1972).
M. Imanary, M. Ochuchi, and K. Ishizu, “13C Spin-lattice relaxation of hindered bi-phenyls with ortho- and para-alkyl substituents,” J. Magn. Reson., 14, No. 3, 374–377 (1974).
R. Freeman, H. D. W. Hill, and B. L. Tomlinson, “Dipolar contribution to NMR spin-lattice relaxation of protons,” J. Chem. Phys., 61, No. 11, 4466–4473 (1974).
G. Pouzard, L. D. Hall, J. P. Zahra, and B. Woegell, “Détermination des déplacements chimiques des protons axiaux équatiaux dans une bromo cyclohexanone. Une application de la mesure des temps de relaxation longitudinaux des protons,” Org. Magn. Reson., 9, No. 11, 627–630 (1977).
Yu. Yu. Samitov and R. H. Sadykov, “Stereospecificity of selective proton relaxation times in rigid organic molecules,” Magn. Reson. and Related Phenomena: Proc. Tenth Congres AMPERE (1979), p. 462.
K. A. Mochammad, “Proton spin-lattice relaxation study N-aryl-1-isoindolinones,” J. Heterocycl. Chem., 17, No. 4, 651–656 (1980).
Yu. Yu. Samitov and R. Kh. Sadykov, Selective Nuclear Magnetic Relaxation Times and the Conformation of Organic Compounds. Conformational Analysis of Heteroorganic Compounds [in Russian], Nauka, Moscow (1983), pp. 262–307.
I. R. Roman, J. A. McCammon, and B. D. Sykes, “A study of the distances obtained from nuclear magnetic resonance nuclear Overhauser effect and relaxation time measurements in organic structure determination. Distance involving internally rotating methyl groups. Application to cis- and trans-crotonaldehyde,” J. Am. Chem. Soc., 96, No. 15, 4773–4777 (1974).
Yu. Yu. Samitov, “Anomalous chemical shifts and conformation of cyclic sulfite and selenite esters,” Dokl. Akad. Nauk SSSR, 164, No. 2, 347–350 (1965).
E. Haslinger and R. M. Lynden-Bell, “Investigation of the internal rotation of methyl groups by T1 relaxation measurements,” J. Magn. Reson., 31, No. 1, 33–40 (1978).
P. S. Hubbard, “Nonexponential relaxation of rotating three-spin systems in molecules of a liquid,” J. Chem. Phys., 52, No. 2, 563–568 (1970).
K. A. Valiev and E. N. Ivanov, “Rotational Brownian motion,” Usp. Fiz. Nauk, 109, No. 1, 31–64 (1973).
C. Altona, H. J. Geise, and C. Romers, “The conformation of nonaromatic ring compounds. 32. The crystal structure of trimethylene sulfite at −100‡C,” Rec. Trav. Chim., 85, No. 11/12, 1197–1205 (1966).
W. M. K. J. Bovee, J. Vriend, J. A. Peters, et al., “NMR relaxation of spin 1/2 nuclei in rigid parts of a molecule by dipolar interaction with reorienting CX3 groups and vice versa: Extension of theory and an application to spectral assignment and determination of barriers to methyl rotation,” Mol. Phys., 41, No. 4, 933–940 (1980).
D. E. Woessner, “Nuclear magnetic dipole-dipole relaxation in molecules with internal motion,” J. Chem. Phys., 42, No. 6, 1855–1859 (1965).
B. A. Arbuzov, E. N. Klimovitskii, and G. N. Sergeeva, “Stereochemistry of seven-membered heterocycles. 7. Synthesis and conformational analysis of diastereomeric 4,7-dimethylbenzo[e]-1,3-dioxepanes,” Zh. Obshch. Khim., 52, No. 11, 2418–2421 (1982).
R. Kh. Sadykov, P. P. Chernov and Yu. Yu. Samitov, Correction of the Recovery of Nuclear Magnetization during Measurements of Longitudinal Relaxation in Single-Coil High-Resolution NMR Spectrometers with Fourier Transformation [in Russian], Moscow (1980), Dep. VINITI, No. 3411-80.
Author information
Authors and Affiliations
Additional information
Translated from Teoreticheskaya i éksperimental'naya Khimiya, Vol. 22, No. 5, pp. 603–610, September–October, 1986.
The authors express their gratitude to Academician B. A. Abruzov for constant interest in the work and to E. N. Klimovitskii for the synthesis of some of the investigated substances.
Rights and permissions
About this article
Cite this article
Samitov, Y.Y., Sadykov, R.K. Stereospecificity in the proton magnetic relaxation rate of enantiotopic methyls and the methyl rotation barrier. Theor Exp Chem 22, 576–583 (1987). https://doi.org/10.1007/BF00522544
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00522544