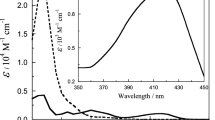
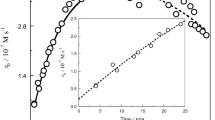
Abstract
The kinetics of hydrogen peroxide oxidation of Fe(II) to Fe(III) complexed with tetraazamacrocyclic ligand was studied, and a decrease in the reaction rate was observed in the presence of nitrogeneous bases, capable of forming hexacoordinated complexes with tetraazamacrocyclic compound of iron(II). The rate of reaction is proprotional to the concentration of the iron complex and hydrogen peroxide and inversely proportional to the concentration of the nitrogeneous base. A mechanism for the course of the reaction has been proposed, and the rate constants of the oxidation of the pentacoordinated iron(II) complexes have been calculated. It was shown that the addition of the fifth donor particle (in particular imidazole) activates the iron(II) atom with respect to the oxidation reaction. It was found that a tetraazamacrocyclic complex of iron(II) is capable of displaying a peroxidase type activity.
Similar content being viewed by others
Literature cited
Inorganic Biochemistry [Russian translation], Vol. 2, G. Eichhorn, editor, Mir, Moscow (1978).
A. C. Melnyk, N. K. Kildahl, A. R. Rendina, et al., “Catalysis of the decomposition of hydrogen peroxide by a complex of iron(III) with a synthetic macrocyclic ligand,” J. Amer. Chem. Soc., 101, No. 12, 3232–3240 (1979).
I. V. Pang and D. V. Stynes, “Kinetics and equilibria for carbon monoxide and benzyl isocyanide binding to ferrous tetrabenzo [b,f,j,b]tetraazacyclotetradecine,” Inorg. Chem., 16, No. 9, 2192–2199 (1977).
V. Katovic, S. C. Vergez, and D. H. Busch, “Mononuclear and oxo-bridged binuclear iron(III) complexes containing the cyclic tetradentate Schiff base derived from o-aminobenzaldehyde,” Inorg. Chem., 16, No. 7, 1716–1720 (1977).
W. T. Oosterhuis, “The electronic state of iron in some natural iron compounds: determination by Mössbauer and ESR Spectroscopy,” Struct. and Bond, 20, 59–100 (1974).
N. M. Émanuél' and D. G. Knorre, Course of Chemical Kinetics [in Russian], Vysshaya Shkola, Moscow (1969).
B. A. Dolgoplosk and E. I. Tinyakova, Oxidation-Reduction Systems as Sources of Free Radicals [in Russian], Nauka, Moscow (1972).
D. P. Rillema, J. F. Endicott, and E. Papaconstantinou, “Oxidation-reduction behavior of complexes containing macrocyclic ligands. An electrochemical comparison of complexes with the metals iron through zinc”, Inorg. Chem., 10, No. 8, 1739–1746 (1971).
D. E. Pennington, “Oxidation-reduction reactions of coordination complexes,” in: Coordination Chemistry, Amer. Chem. Soc., Washington (1978), pp. 476–590.
B. Pulman and A. Pulman, Quantum Biochemistry [Russian translation], Mir, Moscow (1965).
N. K. Kildahl, K. J. Balkus, and M. J. Flynn, “Axial labilization of macrocyclic ligands. 3. Kinetics of axial substitution in iron(II) complexes of two tetraimine macrocyclic ligands,” Inorg. Chem., 22, No. 4, 589–592 (1983).
N. K. Kildahl, G. Antonopoulos, N. E. Fortier, et al., “Axial labilization by macrocyclic ligands. 4. Further studies of axial substitution of low-spin iron(II) complexes containing 14-membered tetraazamacrocyclic ligands”, Inorg, Chem., 24, No. 3, 429–432 (1985).
G. Muller, M. Otto, and G. Werner, Catalytic Methods in Analysis of Trace of Elements [Russian translation], Mir, Moscow (1983).
Author information
Authors and Affiliations
Additional information
Translated from Teoreticheskaya Eksperimental'naya Khimiya, Vol. 22, No. 3, pp. 309–316, May–June, 1986.
Rights and permissions
About this article
Cite this article
Yatsimirskii, K.B., Rybak-Akimova, E.V. Reaction of hydrogen peroxide with tetraazamacrocyclic complex of iron(II) in the presence of nitrogeneous bases. Theor Exp Chem 22, 291–298 (1986). https://doi.org/10.1007/BF00521155
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00521155