Abstract
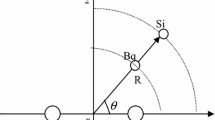
A method has been derived for the analytic description of potential surfaces in natural reaction coordinates as a preliminary stage in researching the dynamics of SN2-type reactions X−+CH3Y→CH3X+Y−; a potential surface is specified by the potential profile V(s) along the reaction path s, the dependence of the frequencies of the transverse vibrations ωs(s) on s, and the path curvature g(s). V(s) and ωs(s) have been constructed from nonempirical calculations in the 4–31G basis for the energies, geometrical parameters, and vibrational frequencies of the reactants, products, transitional states, and prereaction and postreaction complexes. The parameters of g(s) have been chosen from the condition for the reaction path to pass through all the stationary points on the potential surface. This way of describing a surface leaves adequate freedom for correcting the energy parameters by reference to thermochemical measurements, as well as for examining the effects of the parameters on the reaction dynamics.
Similar content being viewed by others
Literature cited
K. Tanaka, G. I. Mackay, J. D. Payzant, and D. K. Bohms, “Gas-phase reactions of anions with halogenated methanes at 297±2 K,” Can. J. Chem., 54, No. 10, 1643–1659 (1976).
W. N. Olmstead and J. I. Brauman, “Gas-phase nucleophilic displacement reactions,” J. Amer. Chem. Soc, 99, No. 13, 4219–4228 (1977).
M. J. Pellerite and J. I. Brauman, “Intrinsic barriers in nucleophilic displacement,” Ibid, 102, No. 19, 5993–5999 (1980).
A. Dedieu and A. Veillard, “A comparative study of some SN2 reactions through ab initio calculations,” Ibid, 94, No. 19, 6730–6738 (1972).
H. B. Schlegel, K. Mislow, F. Bernardi, and A. Bottoni, “An ab initio investigation into SN2 reaction: frontside attack versus backside attack in the reaction of F− with CH3F,” Theor. Chim. Acta., 44, No. 3, 245–256 (1977).
S. Wolfe, D. J. Mitchell, and H. B. Schlegel, “Theoretical studies of SN2 transition states,” J. Amer. Chem. Soc., 103, No. 24, 7692–7694 (1981).
M. Urban, I. Cernusak, and V. Kellö, “Activation barriers of SN2 reactions: F−+CH3F and H−+CH3F. Fourth-order MB RSPT calculations,” Chem. Phys. Lett., 105, No. 6, 625–629 (1984).
C. E. Bloom, P. J. Slingerland, and C. Altona, “Application of self-consistent field ab initio calculations to organic molecules. 1. Equilibrium structure and force constants of hydrocarbons,” Mol. Phys., 31, No. 5, 1359–1376 (1976).
C. E. Bloom, L. P. Otto, and C. Altona, “Application of self-consistent field ab initio calculations to organic molecules. 3. Equilibrium structure of water, methanol and dimethyl ether, general valence field of water and methanol sealed on experimental frequencies,” Ibid, 32, No. 4, 1137–1179 (1976).
R. D. Marcus, “On the analytical mechanics of chemical reactions. Quantum mechanics of linear collisions,” J. Chem. Phys., 45, No. 2, 4493–4500 (1966).
M. V. Basilevsky, “Natural coordinates for polyatomic reactions,” Chem. Phys., 24, No. 1, 81–89 (1977).
W. H. Miller, N. C. Handy, and J. E. Adams, “Reaction path Hamiltonian for polyatomic molecules,” J. Chem. Phys., 72, No. 1, 99–112 (1980).
D. G. Truhlar, W. L. Hase, and J. T. Hynes, “Current status of transition state theory,” ibid, 87, No. 15, 2664–2682 (1983).
J. M. Bowman, G.-Zh. Ju, and K. T. Lee, “Incorporation of collinear exact quantum reaction probabilities into three-dimensional transition state theory,” ibid, 86, No. 12, 2232–2239 (1982).
S. Glasstone, K. Laidler, and H. Eyring, Reaction Rate Theory [Russian translation], Izd. Inert. Lit., Moscow (1948).
C. Eckart, “The penetration of a potential barrier by electrons,” Phya. Rev., 35, No. 11, 1303–1309 (1930).
M. V. Basilevsky and V. M. Ryaboy, “Dynamics of linear exchange reactions. Quasiclassical model of high-energy vibrational inversion,” Chem, Phys., 41, No. 3, 477–488 (1979).
J. C. Polanyi, “Some concepts in reaction dynamics,” Acc. Chem. Research, 5, No. 5, 161–168 (1972).
M. V. Basilevsky and V. M. Ryaboy, “Quantum dynamics of linear triatomic reactions,” Adv. Quant. Chem., 15, 1–83 (1982).
Author information
Authors and Affiliations
Additional information
Translated from Teoreticheskaya i Éksperimental'naya Khimiya, No. 3, pp. 271–280, May–June, 1986.
I am indebted to A, I. Beldyrev for providing the program for the quantum-chemical calculations.
Rights and permissions
About this article
Cite this article
Ryaboi, V.M. Nonempirical calculations on potential surfaces for gas-phase SN2 nucleophilic substitution reactions. Theor Exp Chem 22, 253–260 (1986). https://doi.org/10.1007/BF00521149
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00521149