Abstract
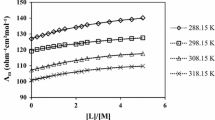
Various experimental methods have been used in a study of the effect of complexation on the electrical conductivity in systems consisting of an anion-radical salt of an alkali metal, a crown ether (CE), and a polar solvent. The electrical conductivity of solutions of M+TCQDM∸ (TCQDM = tetracyanoquinodimethane) in the presence of the CE is determined by the ratio (ℓ) of the radius of the CE cavity to that of the metal ion. The equilibrium constants of the processes taking place in these systems have been determined. It has been established that the electrical conductivity of the systems is determined by the following: a) electrostatic interaction (with ℓ = 0–0.8 and ℓ > 2); b) formation of complexes {M+...CE (with ℓ }- 1); c) formation of ternary associates {A∸...M+...CE (with ℓ }- 1.4). In the last case, the symmetry of the environment of the M+ ion is increased and the potential barrier to the transition of ions between the equilibrium positions is lowered, which is responsible for the observed increase in mobility of the ions in solution.
Similar content being viewed by others
Literature cited
M. P. Eastman, D. A. Ramires, C. D. Jaeger, and M. T. Watts, “Electron spin resonance studies of ion pair complexes involving tetracyanoethylene anion radical,” J. Phys. Chem., 80, No. 2, 182–186 (1976).
H. Kohama, M. Joshinaga, and K. Ishizu, “The dynamical behavior of the sodium cation in some crown-Na+TCNE∸ ternary complexes as studied by ESR,” Bull. Chem. Soc. Jpn., 53, No. 12, 3707–3708 (1980).
Ch. A. Kraus and R. M. Fuoss, “The evaluation of λ0 and K for incompletely dissociated electrolytes,” J. Am. Chem. Soc., 55, No. 2, 476–491 (1933).
A. Weissberger and E. Proskauer, Organic Solvents, 2nd edn., revised by J. A. Riddick and E. E. Toops, Wiley-Interscience, New York (1955).
D. S. Acker, R. J. Harter, W. R. Hertler, et al., “7,7,8,8-Tetracyanoquinodimethane and its electrically conducting anion derivatives,” J. Am. Chem. Soc., 82, No. 24, 6408–6409 (1960).
R. Vollmer and W. Caspary, “Computer analysis of ESR data. Integration for concentration determination and resolution of multiplecomponent spectra,” J. Magn. Reson., 27, No. 1, 181–192 (1977).
F. De Yong and D. N. Reunhoudt, “Stability and reactivity of crown ether complexes,” Adv. Phys. Org. Chem., 18, 279–433 (1980).
J. Takada, H. Jano, M. Ishibashi, and H. Isozumi, “A conductance study of alkali metal ion-15-crown-5 and dibenzo-24-crown-6 complexes in propylene carbonate,” Bull. Chem. Soc. Jpn., 53, No. 1, 72–76 (1980).
A. Pullman, C. Giessner-Prette, and Yu. A. Kruglyak, “Cation binding to crown ethers: An ab initio study,” Chem. Phys. Lett., 35, NO. 2, 156–160 (1975).
N. A. Izmailov, Electrochemistry of Solutions [in Russian], Khimiya, Moscow (1976).
M. Szwarc, ed., Ions and Ion Pairs in Organic Reactions, Wiley, New York (1972–4).
R. Foster, Organic Charge-Transfer Complexes, Academic Press, New York (1969).
K. B. Yatsimirskii, E. E. Kriss, and V. L. Gvyazdovskaya, Stability Constants of Complexes of Metals with Bioligands [in Russian], Naukova Dumka, Kiev (1977).
Yu. Ya. Fialkov and A. N. Zhitomirskii, Physical Chemistry of Inorganic Solutions [in Russian], Khimiya, Leningrad (1973).
D. Bright and M. R. Truter, “Crystal structures of complexes between alkali metal salts and cyclic polyethers,” J. Chem. Soc. B, No. 8, 1544–1545 (1970).
J. Dale and P. O. Kristiansen, “Macrocyclic oligo-ethers related to ethylene oxide,” Acta Chem. Scand., 26, No. 4, 1471–1478 (1972).
A. T. Solomonova, N. T. Berberova, A. A. Kazarov, and O. Yu. Okhlobystin, “Complexes of crown ethers as background electrolytes,” Élektrokhimiya, 20, No. 10, 1326–1329 (1984).
A. J. Gordon and R. A. Ford, The Chemist's Companion, Wiley, New York (1972).
Author information
Authors and Affiliations
Additional information
Translated from Teoreticheskaya i Éksperimental'naya Khimiya, Vol. 22, No. 2, pp. 188–196, March–April, 1986.
Rights and permissions
About this article
Cite this article
Kuts, V.S., Zhalko-Titarenko, O.V. Effect of complexation on electrical conductivity in system consisting of an anion-radical salt and a crown ether. Theor Exp Chem 22, 176–183 (1986). https://doi.org/10.1007/BF00519189
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00519189