Summary
Blastocysts were rinsed out of the uterus by glutaraldehyde on Day 4 and 5 in normally pregnant and in ovariectomized, hormone-treated mice and subsequently prepared for and examined by scanning electron microscopy (SEM).
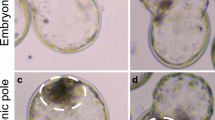
The zona pellucida surface showed no differences in early and late stages, either in normal pregnancy or after ovariectomy and administration of ovarian hormones. In all types of treatment, hatching of the blastocyst from the zona was observed.
The shedding process was oestrogen-dependent and the zona-shedding rate of normal pregnancy could be provoked by 0.01 or 0.1 μg oestradiol-17 β given either late on Day 3 or early on Day 4 but not early on Day 3. A subimplantation dose of oestrogen (0.001 μg) did not seem to influence the shedding process, nor did 0.1 μg if pretreatment with progesterone was omitted after ovariectomy.
The zona-free blastocyst in normal pregnancy usually showed signs of abembryonic proliferation and sometimes detachment injuries in the abembryonic pole, indicating incipient implantation. Similar appearances were noted in blastocysts from progesterone-maintained, ovariectomized mice given 0.01 or 0.1 μg oestradiol-17 β early on Day 4 but not on Day 3. In progesterone-maintained, ovariectomized mice given the above oestrogen doses on Day 3, the zona-free blastocysts were similar to those observed in corresponding animals not treated with oestrogen, i. e. without signs of incipient implantation at any stage. A subimplantation dose of oestrogen (0.001 μg) had no visible effect on the surface of the zona-free blastocyst.
It is concluded that hatching occurs under normal conditions and can be provoked by various hormonal treatments. Further, the location or maturity of the morulae may account for the lack of oestrogen response in zygotes early on Day 3. Since a zona-shedding curve close to the normal one was obtained after oestrogen treatment that did not induce implantations, it is inferred that the oestrogen surge may not constitute a discrete peak.
Similar content being viewed by others
References
Bergström, S.: Surface ultrastructure of mouse blastocysts before and at implantation. I. Preparation of blastocysts for scanning electro-microscopy. In press, 1971 a.
Bergström, S.: Surface ultrastructure of mouse blastocysts before and at implantation. III. Delay of implantation by ovariectomy or lactation. In press, 1971 b.
Bergström, S.: Surface ultrastructure of mouse blastocysts before and at implantation. IV. Induction of implantation by oestrogen in experimentally delayed implantation. In press, 1971 c.
Bergström, S.: Unpublished data, 1971 d.
Bergström, S.: Evidence for hatching of the zona pellucida of the mouse blastocyst in normal pregnancy. Submitted to J. Reprod. Fertil., 1971 e.
Bergström, S., Nilsson, O.: Ultrastructural response of blastocysts and uterine epithelium upon a deprivation of progesterone during delayed implantation in the mouse. Submitted to J. Endocr., 1972 a.
Bergström, S., Nilsson, O.: Scanning and transmission electron microscopy of various embryoendometrial contacts during delay of implantation in the mouse. Ups. J. Med. Sci. In press, 1972 b.
Bindon, B. M., Lamond, D. R.: Effect of hypophysectomy on implantation in the mouse. J. Reprod. Fertil. 18, 43–50 (1969).
Bitton-Casimiri, V., Psychoyos, A.: Développment du blastocyste du Rat in vitro. C. R. Acad. Sci. (Paris) 267, 762–764 (1968).
Bloch, S.: Enhancement of on-time nidations in suckling mice by the proximity of strange males. J. Endocr. 49, 431–436 (1971).
Bowman, P., McLaren, A.: Viability and growth of mouse embryos after in vitro culture and fusion. J. Embryol. exp. Morph. 23, 693–704 (1970a).
Bowman, P., McLaren, A.: The reaction of the mouse blastocyst and its zona pellucida to enzymes in vitro. J. Embryol. exp. Morph. 24, 331–334 (1970b).
Bruce, H. M.: A block to pregnancy in the mouse caused by the proximity of strange males. J. Reprod. Fertil. 1, 96–103 (1960).
Bruce, H. M.: Olfactory block to pregnancy among grouped mice. J. Reprod. Fertil. 6, 451–460 (1963).
Bryson, D. L.: Development of mouse eggs in diffusion chambers. Science 144, 1351–1353 (1964).
Cole, R. J., Paul, J.: Properties of cultured preimplantation mouse and rabbit embryos and cell strains derived from them. In: Preimplantation stages of pregnancy (ed. G. E. W. Wolsteholme). p. 82–122. London 1965.
Cole, R. J.: Cinemicrographic observations on the trophoblast and zona pellucida of the mouse blastocyst. J. Embryol. exp. Morph. 17, 481–490 (1967).
Dickmann, Z.: Shedding of the zona pellucida by the rat blastocyst. J. exp. Zool. 165, 127–138 (1967).
Dickmann, Z.: Shedding of the zona pellucida. In: Advances in reproductive physiology IV (ed. A. McLaren), p. 187–206. London 1969.
Dickmann, Z., DeFeo, V. J.: The rat blastocyst during normal pregnancy and during delayed implantation, including an observation on the shedding of the zona pellucida. J. Reprod. Fertil. 13, 3–9 (1967).
Dickmann, Z., Noyes, R. W.: The zona pellucida at the time of implantation. Fertil. and Steril. 12, 310–318 (1961).
Dickson, A. D.: The form of the mouse blastocyst. J. Anat. (Lond.) 100, 335–348 (1966).
Dickson, A. D.: Variations in development of mouse blastocysts. J. Anat. (Lond.) 101, 263–267 (1967).
Dominic, C. J.: The origin of the pheromones causing pregnancy block in mice. J. Reprod. Fertil. 10, 469–472 (1965).
Fawcett, D. W., Wislocki, G. B., Waldo, C. M.: The development of mouse ova in the anterior chamber of the eye and in the abdominal cavity. Amer. J. Anat. 81, 413–443 (1947).
Finn, C. A., Martin, L.: Hormone secretion during early pregnancy in the mouse. J. Endocr. 45, 57–65 (1969).
Follett, E. A. C., Goldman, R. D.: The occurrence of microvilli during spreading and growth of BHK 21/C 13 fibroblasts. Exp. Cell Res. 59, 124–136 (1970).
Harter, B. T.: Glycogen and carbohydrate-protein complexes in the ovary of the white rat during the oestrus cycle. Anat. Rec. 102, 349–364 (1948).
Humphrey, K. W.: Induction of implantation of blastocysts transferred to ovariectomized mice. J. Endocr. 44, 299–305 (1969).
Kraicer, P. F.: Studies on the mechanism of nidation. XXIV. Isolation and study of intrauterine ova from the rat: technique and observations. Int. J. Fertil. 12, 320–328 (1967).
Loewenstein, J. E., Cohen, A. I.: Dry mass, lipid content and protein content of the intact and zona-free mouse ovum. J. Embryol. exp. Morph. 12, 113–121 (1964).
McLaren, A.: Delayed loss of the zona pellucida from blastocysts of suckling mice. J. Reprod. Fertil. 14, 159–162 (1967).
McLaren, A.: A study of blastocysts during delay and subsequent implantation in lactating mice. J. Endocr. 42, 453–463 (1968).
McLaren, A.: Can mouse blastocysts stimulate a uterine response before losing the zona pellucida? J. Reprod. Fertil. 19, 199–201 (1969).
McLaren, A.: The fate of the zona pellucida in mice. J. Embryol. exp. Morph. 23, 1–19 (1970).
Meunier, J. M., Thevenot-Duluc, A. J., Mayer, G.: Evolution de la zone pellucide des blastocystes de Ratte en fonction des condition hormonales. C. R. Soc. Biol. (Paris) 159, 1643–1646 (1966).
Miller, B. G., Owen, W. H., Emmens, C. W.: The incorporation of tritiated uridine in the uterus and vagina of the mouse during early pregnancy. J. Endocr. 41, 189–195 (1968).
Mintz, B.: Experimental study of the developing mammalian egg: removal of the zona pellucida. Science 138, 594–595 (1962).
Nilsson, O.: Unpublished data, 1971.
Orsini, M. W., McLaren, A.: Loss of the zona pellucida in mice and the effect of tubal ligation and ovariectomy. J. Reprod. Fertil. 13, 483–499 (1967).
Paterson, A. W.: The loss of the zona pellucida of the mouse. Thesis. Bangor 1964.
Potts, D. M., Psychoyos, A.: L'ultrastructure des relations ovo-endométriales au cours du retard de nidation chez la Souris. C. R. Acad. Sci. (Paris) 264, 956–958 (1967).
Potts, D. M., Wilson, I. B.: The preimplantation conceptus of the mouse at 90 hours post coitum. J. Anat. (Lond.) 102, 1–11 (1967).
Psychoyos, A.: Influence of oestrogen on the loss of the zona pellucida in the rat. Nature (Lond.) 211, 864 (1966).
Reinius, S.: Ultrastructure of blastocyst attachment in the mouse. Z. Zellforsch. 77, 257–266 (1967).
Reinius, S.: Morphology of oviduct, gametes and zygotes as a basis of oviductal function in the mouse. Thesis. Uppsala 1969.
Restall, B. J., Bindon, B. M.: The timing and variation of preimplantation events in the mouse. J. Reprod. Fertil. 24, 423–426 (1971).
Rumery, R. E., Blandau, R. J.: The loss of the zona pellucida in delayed implantation. Anat. Rec. 154, 485–486 (1966).
Rumery, R. E., Blandau, R. J.: Loss of zona pellucida and prolonged gestation in delayed implantation in mice. In: The biology of the blastocyst (ed. R. J. Blandau), p. 115–129. New York 1971.
Runner, M. N.: Development of mouse eggs in the anterior chamber of the eye. Anat. Rec. 98, 1–17 (1947).
Schlafke, S., Enders, A. C.: Cytological changes during cleavage and blastocyst formation in the rat. J. Anat. (Lond.) 102, 13–32 (1967).
Shaikh, A. A., Abraham, G. E.: Measurement of estrogen surge during pseudopregnancy in rats by radioimmunoassay. Biol. Reprod. 1, 378–380 (1969).
Shelesnyak, M. C.: Nidation of the fertilized ovum. Endeavour 19, 81–86 (1960).
Shelesnyak, M. C., Kraicer, P. F., Zeilmaker, G. H.: Studies on the mechanism of decidualization. I. The oestrogen surge of pseudopregnancy and progravidity and its role in the process of decidualization. Acta endocr. (Kbh.) 42, 225–232 (1963).
Smith, D. M.: The effect of the time of oestrogen injection on implantation in ovariectomized pregnant mice. J. Endocr. 41, 11–15 (1968).
Szabo, K. T., Free, S. M., Birkhead, H. A., Gay, P. E.: Predictability of pregnancy from various signs of mating in mice and rats. Lab. Anim. Care 19, 822–825 (1969).
Whitten, W. K.: The effect of progesterone on the development of mouse egg in vitro. J. Endocr. 16, 80–85 (1957).
Yasukawa, J. J., Meyer, R. K.: Effect of progesterone and oestrone on the preimplantation and implantation stages of embryo development in the rat. J. Reprod. Fertil. 11, 245–255 (1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bergström, S. Shedding of the zona pellucida in normal pregnancy and in various hormonal states in the mouse. Z. Anat. Entwickl. Gesch. 136, 143–167 (1972). https://doi.org/10.1007/BF00519175
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00519175