Abstract
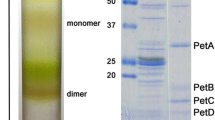
Two soluble c-type cytochromes (c-553 and c-555) and the nonheme iron-containing protein rubredoxin of the non-thiosulfate-utilizing green sulfur bacterium Pelodictyon luteolum were highly purified by ion exchange column chromatography, gel filtration and ammonium sulfate fractionation. Both cytochrome are small and basic hemoproteins, while rubredoxin is an acidic small nonheme iron protein. Cytochrome c-553 has a molecular weight of 13,000 determined by Sephacryl S-200 chromatography and of 10,700 by electrophoresis on SDS acrylamide gel, an isoelectric point at pH 10.2, a redox-potential of +220 mV. It shows maxima at 413 nm in the oxidized form, and the characteristic three maxima in the reduced state (α-band at 553 nm, β-band at 523 nm, γ-band at 417 nm). The best purity index (A 280/A 417) obtained was 0.18. Cytochrome c-555 (best purity index obtained: A 280/A 418=0.17) has an isoelectric point at pH 10.5, a molecular weight of 9,500 (by electrophoresis on SDS acrylamide gel) and a redox-potential of +160mV. The reduced form of this cytochrome shows the typical bands of c-type cytochromes at 555 (551) nm (α-band), 523 nm (β-band) and 418 nm (γ-band), while the oxidized form has the γ-band at 413 nm.
Rubredoxin (best purity index obtained: A 280/A 490=3.5) is an acidic small protein. Its molecular weight estimated by gel filtration and SDS acrylamide gel electrophoresis is 27,000 and 6,300 respectively. The monomer of this protein contains one iron atom per molecule. Rubredoxin has an isoelectric point at pH 2.8 and shows maxima at 570 nm, 490 nm and 370 nm in the oxidized form.
During anaerobic sulfide oxidation of a growing culture of Pelodictyon luteolum elemental sulfur is the first main product, which appears in the medium. Elemental sulfur is further oxidized to sulfate, after the available sulfide is completely consumed by the cells.
Similar content being viewed by others
Abbreviations
- C:
-
Chlorobium
- P:
-
Pelodictyon
- SDS:
-
sodium dodecylsulfate
- HIPIP:
-
high-potential-iron-sulfur-protein Offprint requests to: U. Fischer
References
Bartlett JK, Skoog DA (1954) Colorimetric determination of elemental sulfur in hydrocarbons. Anal Chem 26:1003–1011
Bartsch RG (1971) Cytochromes. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 23. Academic Press, New York, pp 344–363
Dodgson KS (1961) Determination of inorganic sulphate in studies on the enzymic and non-enzymic hydrolysis of carbohydrate and other sulphate esters. Biochem J 78:312–319
Fischer U (1977) Die Rolle von Cytochromen im Schwefelstoffwechsel phototropher Schwefelbakterien. Doctoral thesis, Univ Bonn
Fukumori Y, Yamanaka T (1979) A high potential nonheme iron protein (HIPIP)-linked, thiosulfate-oxidizing enzyme derived from Chlorobium thiosulfatophilum) in oxidation of thiosulfate. Biochem Biophys Res Comm 51:107–112
Kusai A, Yamanaka T (1973b) The oxidation mechanism of thiosulfate and sulfide in Chlorobium thiosulfatophilum. Roles of cytochrome c-551 and cytochrome c-553. Biochim Biophys Acta 325:304–314
Kusai A, Yamanaka T (1973c) Cytochrome c (553,Chlorobium thiosulfatophilum) is a sulfide-cytochrome c reductase. FEBS Lett 34:235–237
Lovenberg W (1972) Clostridial rubredoxin. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 24. Academic Press, New York London, pp 477–480
Lovenberg W, Walker MN (1978) Rubredoxin. In: Colowick SP,Kaplan NO (eds) Methods in enzymology, vol 53. Academic Press, New York San Francisco London, pp 340–346
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin reagent. J Biol Chem 193:265–275
Mathewson JH, Burger LJ, Millstone HG (1968) Cytochrome c-551: thiosulfate oxidoreductase from Chlorobium thiosulfatophilum. Fed Proc 27:774
Meyer TE, Bartsch RG, Cusanovich MA, Mathewson JH (1968) The cytochromes of Chlorobium thiosulfatophilum. Biochim. Biophys Acta 153:854–861
Meyer TE, Sharp JJ, Bartsch RG (1971) Isolation and properties of rubredoxin from the photosynthetic green sulphur bacteria. Biochim Biophys Acta 234:266–269
Moriarty DJW, Nicholas DJD (1970) Electron transfer during sulphide and sulphite oxidation by Thiobacillus concretivorus. Biochim Biophys Acta 216:130–138
Pachmayr F (1960) Vorkommen und Bestimmung von Schwefelverbindungen in Mineralwasser. Doctoral thesis, Univ Munich
Pfennig N (1965) Anreicherungskulturen für rote und grüne Schwefelbakterien. In: Schlegel HG, Kröger E (eds) Anreicherungskulturen und Mutantenauslese. Fischer, Stuttgart, pp 179–189, 503–504
Pfennig N, Lippert KD (1966) Über das Vitamin B12-Bedürfnis phototropher Schwefelbakterien. Arch Mikrobiol 55:245–256
Schedel M (1978) Untersuchungen zur anaeroben Oxidation reduzierter Schwefelverbindungen durch Thiobacillus denitrificans, Chromatium vinosum und Chlorobium limicola. Doctoral thesis, Univ Bonn
Schleifer G, Schmitt W, Knobloch K (1981) The enzymatic system thiosulfate: cytochrome c oxidoreductase from photolithoautotrophically grown Rhodopseudomonas palustris. Arch Microbiol 130:328–333
Schmitt W, Schleifer G, Knobloch K (1981) The enzymatic system thiosulfate: cytochrome c oxidoreductase from photolithoautotrophically grown Chromatium vinosum. Arch Microbiol 130: 334–338
Steinmetz MA, Fischer U (1981) Cytochromes of the non-thiosulfateutilizing green sulfur bacterium Chlorobium limicola. Arch Microbiol 130:31–37
Steinmetz MA, Fischer U (1982) Cytochromes of the green sulfur bacterium Chlorobium vibrioforme f. thiosulfatophilum. Purification, characterization and sulfur metabolism. Arch Microbiol 131:19–26
Trüper HG, Schlegel HG (1964) Sulphur metabolism in Thiorhodaceae. I. Quantitative measurements on growing cells of Chromatium okenii. Antonie van Leeuwenhoek J Microbiol Serol 30:225–238
Urban PJ (1961) Colorimetry of sulfur anions. Part I. An improved colorimetric method for determination of thiosulfate. Z Analyt Chem 179:415–422
Weber K, Osborne M (1969) The reliability of molecular weight determinations by dodecylsulfate-polyacrylamide-gel electrophoresis. J Biol Chem 244:4406–4412
Winter A, Ek K, Andersson UB (1977) Analytical electrofocusing in thin layers of polyacrylamide gels. Application Note, No. 250 Methodological, LKB-Produkter AB
Yamanaka T, Fukumori Y, Okunuki K (1979) Preparation of subunits of flavocytochromes c derived from Chlorobium limicola f. thiosulfatophilum and Chromatium vinosum. Anal Biochem 95:209–213
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Steinmetz, M.A., Fischer, U. Cytochromes, rubredoxin, and sulfur metabolism of the non-thiosulfate-utilizing green sulfur bacterium Pelodictyon luteolum . Arch. Microbiol. 132, 204–210 (1982). https://doi.org/10.1007/BF00508732
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00508732