Summary
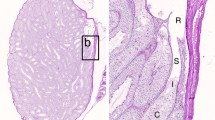
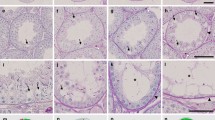
The stages of spermatogenesis can be identified in freshly isolated, unstained adult mouse seminiferous tubules using a transillumination method. Late acrosome- and maturation phase spermatids, arranged in bundles at stages XII–VI give rise to a spotty transillumination pattern. Before spermiation, these cells form a continuous layer on the top of the seminiferous epithelium, recognized by a strong homogeneous central light absorption in the freshly isolated seminiferous tubules at stages VII and VIII. Other stages have a pale light absorption pattern. The accurate determination of the developmental stages of the germ cells was based on the morphology of the developing acrosomic system and of the nuclei of the spermatids, as revealed by phase contrast microscopy. Using this procedure, the activity levels of DNA polymerases α and β have been studied by autoradiography of squash preparations. Using endogenous templates, assay conditions that differentiate between the solubilized DNA polymerases α and β in vitro, were used to distinguish between these activities in situ in different stages of mouse spermatogenesis. Except in very late spermatids shortly before spermiation, DNA polymerases α and β were detectable in all cell types examined. Coinciding with the nuclear protein transitions, elongating spermatids at steps 10–12 and maturation phase spermatids at steps 13–14 showed high DNA polymerase activities. As no replication occurs in these cells, the observations support the view that both DNA polymerases α and β could be involved in repair DNA synthesis.
Similar content being viewed by others
References
Calvin HI, Kosto B, Williams-Ashman HG (1967) Enzymic incorporation of deoxyribonucleosides into DNA in mammalian testes. Arch Biochem Biophys 118:670–681
Ciarrocchi G, Jose JG, Linn S (1979) Further characterization of a cell-free system for measuring replicative and repair DNA synthesis with cultured human fibroblasts and ecidence for the involvement of DNA polymerase α in DNA repair. Nucleic Acids Res 7:1205–1219
Edenberg HJ, Anderson S, DePamphilis ML (1978) Involvement of DNA polymerase α in Simian virus 40 DNA replication. J Biol Chem 253:3273–3280
Grimes SR Jr, Meistrich ML, Platz RD, Hnilica LS (1977) Nuclear protein transitions in rat testis spermatids.Exp Cell Res 110:31–39
Grippo P, Geremia R, Locorotondo G, Monesi V (1978) DNA-dependent DNA polymerase species in male germ cells of the mouse. Cell Differ 7:237–248
Hecht NB, Farrell D, Davidson D (1976) Changing DNA polymerase activities during the development of the testis in the mouse. Dev Biol 48:56–66
Hecht NB, Farrell D, Williams JL (1979) DNA polymerases in mouse spermatogenic cells separated by sedimentation velocity. Biochim Biophys Acta 561:358–368
Hecht NB, Parvinen M (1981) DNA synthesis catalyzed by endogenous templates and DNA-dependent DNA polymerases in spermatogenic cells from rat. Exp Cell Res (in press)
Hobart PM, Infante AA (1978) A low molecular weight DNA polymerase β in the sea urchin, Strongylocentrotus purpuratus. J Biol Chem 253:8229–8238
Ikegami S, Taguchi T, Oguro M, Nagano H, Mano Y (1978) Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase α. Nature 275:458–460
Kofman-Alfaro S, Chandley AC (1970) Meiosis in the male mouse. An autoradiographic investigation. Chromosoma 31:404–420
Leblond CP, Clermont Y (1952) Definition of the stages of the cycle of the siminferous epithelium in the rat. Ann NY Acad Sci 55:548–573
Meistrich ML, Reid BO, Barcellona WJ (1975) Meiotic DNA synthesis during mouse spermatogenesis. J Cell Biol 64:211–222
Monesi V (1962) Autoradiographic study of DNA synthesis and the cell cycle in spermatogonia and spermatocytes of mouse testis using tritiated thymidine. J Cell Biol 14:1–18
Monesi V (1965) Synthetic activities during spermatogenesis in the mouse. RNA and protein. Exp Cell Res 39:197–224
Oakberg EF (1956) A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am J Anat 99:391–413
Otto B, Fanning E (1978) DNA polymerase α is associated with replicating SV 40 nucleoprotein complexes. Nucleic Acids Res 5:1715–1728
Parvinen M, Marana R, Robertson DM, Hansson V, Ritzén EM (1980) Functional cycle of rat Sertoli cells: Differential binding and action of follicle stimulating hormone at various stages of the spermatogenic cycle. In: Steinberger A, Steinberger E (eds) Testicular development, structure and function. Raven Press, New York, pp 425–432
Parvinen M, Vanha-Perttula T (1972) Identification and enzyme quantitation of the stages of the seminiferous epithelial wave in the rat. Anat Rec 174:435–450
Philippe M, Chevaillier P (1980) Study of dogfish (Schyliohinus caniculus) DNA polymerases α and β (extraction, separation, characterization, and changes during spermatogenesis). Biochem J 189:635–639
Ritzén EM, Boitani C, Parvinen M (1981) Cyclic secretion of proteins by the rat seminiferous tubule, depending on the stage of spermatogenesis. Int J Androl (Suppl) 3:57–58
Rogers AW (1969) Techniques of autoradiography. Elsevier, Amsterdam, pp 253–272
Sega GA (1979) Unscheduled DNA synthesis (DNA repair) in the germ cells of male mice—its role in the study of mammalian mutagenesis. Genetics 92:49–58
Söderström K-O, Parvinen M (1976a) RNA synthesis in different stages of rat seminiferous epithelial cycle. Mol Cell Endocrinol 5:181–199
Söderström K-O, Parvinen M (1976b) RNA synthesis during male meiotic prophase in the rat. Hereditas 82:25–28
Villani G, Spadari S, Boiteux S, Defais M, Caillet-Fauquet P, Radman M (1978) Replication of chemically modified DNA. Biochimie 60:1145–1150
Weissbach A (1979) The functional roles of mammalian DNA polymerase. Arch Biochem Biophys 198:386–396
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Parvinen, M., Hecht, N.B. Identification of living spermatogenic cells of the mouse by transillumination-phase contrast microscopic technique for ‘in situ’ analyses of DNA polymerase activities. Histochemistry 71, 567–579 (1981). https://doi.org/10.1007/BF00508382
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00508382