Summary
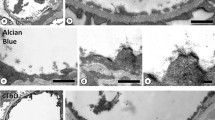
In rabbit luteal cells embedded in glycolmethacrylate and stained with PTA at low pH highly glycosylated membrane patches can be observed after vesiculation of the trans-Golgi network. As these membranes could be prelysosomal, their sialic acid content was investigated by postembedding labeling with Limax flavus agglutinin (LFA)/fetuin-Au. Additional labeling of the Golgi apparatus was performed with Wheat germ agglutinin (WGA)/ovomucoid Au, Ricinus communis agglutininI (RCAI)/Au and Helix pomatia agglutinin (HPA)/Au. The sections were then counterstained with PTA at low pH, which allows a clear distinction between the elements of the trans-Golgi network (G2-G1) and the saccules of the stack (g).
With WGA, LFA and RCAI the trans-Golgi network was observed to be clearly more reactive than the stack. After vesiculation most intense labeling was found over the highly glycosylated vacuolar membranes derived from the G2-element. The limiting membrane of lysosomes, the MvB's and the plasma membrane also reacted strongly. Colloidal gold particles were also found over the membranes of the vacuoles derived from G1. The Golgi stack showed a lower reactivity and label for all three lectins could be found over three to four saccules of the stack (g3-g4). The matrix of the lysosomes was slightly labeled. Labeling with HPA was absent from the trans saccules and was consistently found in the cis and cis-most (g4-g5) saccules of the stack. Some cytoplasmic vesicles near the cell border were also labeled. With our procedure the Golgi apparatus can easily be detected and it is apparent that in rabbit luteal cells the highest lectin reactivity is found in the trans-Golgi network. A striking similarity is observed between the highly glycosylated membrane structures derived from G2 and the border of the lysosomes.
Similar content being viewed by others
References
Berger EG, Hesford FJ (1985) Localization of galactosyl- and sialyltransferase by immunofluorescence: Evidence for different sites. Proc Natl Acad Sci USA 82:4736–4739
Berger EG, Thurnher M, Müller U (1987) Galactosyltransferase and sialyltransferase are located in different subcellular compartments in HeLa cells. Exp Cell Res 173:267–273
Dunphy WG, Brands R, Rothman JE (1985) Attachment of terminal N-acetylglucosamine to asparagine-linked oligosaccharides occurs in central cisternae of the Golgi stack. Cell 40:463–472
Geoghegan WD, Ackerman GA (1977) Absorption of horseradish peroxidase, ovomucoid and anti-immunoglobulin to colloidal gold for the indirect detection of concanavalin A, wheat germ agglutinin and goat-antihuman immunoglobulin G on cell surfaces at the electron microscopic level: a new method, theory and application. J Histochem Cytochem 25:1187–1200
Geuze HJ, Slot JW, Strous GJAM, Hasilik A, Von Figura K (1985) Possible pathways for lysosomal enzyme delivery J Cell Biol 101:2253–2262
Griffiths G, Simons K (1986) The trans Golgi network: Sorting at the exit site of the Golgi complex. Science 234:438–443
Griffiths G, Brands R, Burke B, Louvard D, Warren G (1982) Viral membrane protein acquires galactose in trans Golgi cisternae during intracellular transport. J Cell Biol 95:781–792
Griffiths G, Quinn P, Warren G (1983) Dissection of the Golgi complex. I. Monensin inhibits the transport, of viral membrane proteins from the medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki forest virus. J Cell Biol 96:835–850
Hedman K, Pastan I, Willingham MC (1986) The organelles of the trans domain of the cell. Ultrastructural localization of sialoglycoconjugates using Limax flavus agglutinin. J Histochem Cytochem 34:1069–1077
Horisberger M (1985) The gold method as applied to lectin cytochemistry in transmission and scanning electron microscopy. In: Bullock GR, Petruz P (eds) Techniques in immunocytochemistry, vol 3. Academic Press, London, pp 155–178
Leduc EH, Bernhard W (1967) Recent modification of the glycolmethacrylate embedding procedure. J Ultrastruct Res 19:196–199
Lewis V, Green SA, Marsh M, Vikho P, Helenius A, Mellman I (1985) Glycoproteins of the lysosomal membrane. J Cell Biol 100:1839–1847
Pavelka M, Ellinger A (1985) Localization of binding sites for Concanavalin A, Ricinus communis I and Helix pomatia lectin in the Golgi apparatus, of rat small intestinal absorptive cells. J Histochem Cytochem 33:905–914
Pavelka M, Ellinger A (1987) The Golgi apparatus in the acinar cells of the developing embryonic pancreas: II. Localization of lectin-binding sites. Am J Anat 178:224–230
Quatacker JR (1979) Different aspects of membrane differentiation at the inner side (GERL) of the Golgi apparatus in rabbit luteal cells. Histochem J 11:399–416
Quatacker JR (1987a) On the heterogeneous glycosylation of the membranes of the trans Golgi network in rabbit luteal cells. Histochemistry 87:385–391
Quatacker JR (1987b) Counterstaining of post-embedding lectingold labeled glycolmethacrylate sections with phosphotungstic acid at low pH. Micron Microsc Acta 18:207–208
Roth J (1984) Cytochemical localization of terminal N-acetyl-d-galactosamine residues in cellular compartments of intestinal goblet cells: Implication for topology of O-glycosylation. J Cell Biol 98:399–406
Roth J, Lucocq JM, Berger EG, Paulson JC, Watkins WM (1984a) Terminal glycosylation is compartmentalized in the Golgi apparatus. J Cell Biol 99:229a
Roth J, Lucocq JM, Charest PM (1984b) Light and electron microscopic demonstration of sialic acid residues with the lectin from Limax flavus: A cytochemical affinity technique with the use of a fetuin-gold complex. J Histochem Cytochem 32:1167–1176
Roth, J, Taatjes DJ, Lucocq JM, Weinstein J, Paulson JC (1985) Demonstration of an extensive trans-tubular network continuous with the Golgi apparatus stack that may function in glycosylation. Cell 43:287–295
Roth J, Taatjes DJ, Weinstein J, Paulson JC, Greenwell P, Watkins WM (1986) Differential subcompartmentation of terminal glycosylation in the Golgi apparatus of intestinal absorptive and goblet cells. J Biol Chem 261:14307–14312
Slot JW, Geuze HJ (1983), Immuno electron microscopic exploration of the Golgi complex. J Histochem Cytochem 31:1049–1056
Slot JW, Geuze HJ (1985) A new method of preparing gold probes for multiple labeling cytochemistry. Eur J Cell Biol 38:87–93
Snider MD, Rogers OC (1986) Membrane traffic in animal cells: Cellular glycoproteins return to the site of Golgi mannosidase I. J Cell Biol 103:265–275
Tartakoff A, Vassalli P (1983) Lectin binding sites as markers of Golgi subcompartments proximal-to-distal maturation of oligosaccharides. J Cell Biol 97:1243–1248
Woods JW, Doriaux M, Farquhar MG (1986) Transferrin receptors recycle to cis and middle as well as trans Golgi cisternae in Ig-secreting myeloma cells. J Cell Biol 103:277–286
Yuan L, Barriocanal JG, Bonifacino JS, Sandoval IV (1987) Two integral membrane proteins located in the cis-middle and trans-part of the Golgi system acquire sialylated N-linked carbohydrates and display different turnovers and sensitivity to cAMP-dependent phosphorylation. J Cell Biol 105:215–227
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Quatacker, J.R. Lectin-gold cytochemistry of the Golgi apparatus in rabbit luteal cells, with special emphasis on the formation of a lysosomal-type membrane. Histochemistry 90, 399–404 (1989). https://doi.org/10.1007/BF00508319
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00508319