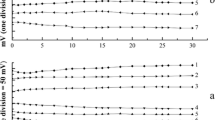
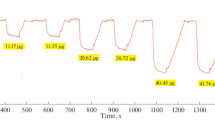
Summary
A method has been developed for the direct determination of microgram amounts of sulphide by means of constant-current coulometric argentometry in ammoniacal-alkaline electrolytes. Constant-current bipotentiometric and biamperometric end-point detection techniques with two identical silver sulphide-silver microelectrodes have been used. The influence of the composition of the supporting electrolyte on the titration efficiency has also been investigated. A 100% titration efficiency is obtainable in electrolytes based upon 0.1 m sodium hydroxide at concentrations of ammonia equal to or greater than 0.1 m. This method permits the determination of 3–100 Μg of sulphide in a sample volume up to 10 ml with an error of ±1%.
Zusammenfassung
Zur Bestimmung von Mikromengen Sulfid wurde eine coulometrische Methode entwickelt, bei der Sulfid in einer Ammoniak und Natronlauge enthaltenden Lösung mit an einer Silberelektrode elektrolytisch bei konstantem Strom erzeugtem Silber titriert wird. Zur Indikation werden zwei Silbersulfid-Silber-Elektroden angewendet; der Endpunkt wird entweder bipotentiometrisch (erzwungener Strom 0,5 ΜA) oder biamperometrisch (angelegte Spannung 100 mV) ermittelt. Die Gesamttitrationsausbeute hÄngt von der Ammoniakkonzentration ab; in 0,1 m Natronlauge erreicht sie 100% bei Ammoniakkonzentrationen oberhalb 0,1 m. In einem Probevolumen von 10 ml können 3–100 Μg Sulfid mit einem Fehler von ±1% titriert werden.
Similar content being viewed by others
References
čaderský, I., and M. PŘibyl: Z. Anal. Chem. 217, 252 (1966).
Craig, D. N., J. I. Hoffman, C. A. Law, and W. J. Hamer: J. Res. Nat. Bur. Std. 64A, 381 (1960).
Dutoit, P., and G. von Weisse: J. Chim. Phys. 9, 578, 608, 630 (1911).
Kiss, S. A.: Z. Anal. Chem. 188, 341 (1962).
Kolthoff, I. M., and E. J. Verzijl: Rec. Trav. Chim. 42, 1055 (1923).
Liu, C. H., and S. Shen: Anal. Chem. 36, 1652 (1964).
Novák, K., and V. Slavík: Czechoslovak Pat. 109043.
Sack, W.: Z. Anal. Chem. 131, 199 (1950).
Tamele, M. W., V. C. Irvine, and L. B. Ryland: Anal. Chem. 32, 1002 (1960); cf. Z. Anal. Chem. 180, 201 (1961).
Treadwell, W. D., and L. Weiss: Helv. Chim. Acta 2, 680 (1919).
Willard, H. H., and F. Fenwick: J. Am. Chem. Soc. 45, 645 (1923).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
čaderský, I., PŘibyl, M. Coulometric argentometry of microgram amounts of sulphide. Z. Anal. Chem. 217, 259–264 (1966). https://doi.org/10.1007/BF00508161
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00508161