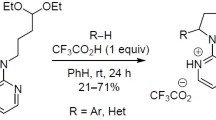
Abstract
Pyrazolo[4,5d]-1,2,3-triazoles and 3,4-dimethyl-1,6-diphenyl-dipyrazolo[4,5-b∶4′,5′- c]-1,6-dihydropyridazine, the structures of which are confirmed by the mass and PMR spectra, were isolated as side products in the preparation of macrocyclic compounds by the template synthesis of o-amino-o'-haloazopyrazoles. The PMR spectra of pyrazolo[4,5-d]-1,2,3-triazoles are examined in comparison with the PMR spectra of the corresponding noncyclic azopyrazoles. All of the compounds obtained were characterized by the results of elementary analysis and data from the IR, UV, mass, and NMR spectra.
Similar content being viewed by others
Literature cited
M. P. Schmidt and A. Hagenböcker, Chem., Ber., 54, 2191 (1921).
A. Muzik, Chem. Listy, 48, 221 (1954).
S. Renson, Chem. Rev., 46, 1 (1950).
V. M. Dziomko, B. K. Berestevich, A. V. Kessenikh, Yu. S. Ryabokobylko, and R. S. Kuzanyan, Khim. Geterotsikl. Soedin., No. 8, 1097 (1978).
V. M. Dziomko, B. K. Berestevich, A. V. Kessenikh, Yu. S. Ryabokobylko, and R. S. Kuzanyan, Khim. Geterotsikl. Soedin., No. 5, 701 (1979).
E. Mohr, J. Prakt. Chem., 79, 1 (1909).
I. I. Grandberg and G. V. Klyuchko, Zh. Obshch. Khim., 32, 1898 (1962).
H. Dorn, J. Prakt. Chem. 315, 382 (1973).
I. S. Shmeleva, N. A. Klyuev, F. L. Kolodkin, R. A. Khmel'nitskii, and I. I. Levkoev, Zh. Org. Khim., 12, 249 (1976).
R. S. Kuzanyan, R. S. Poponova, and M. S. Chupakhin, Zh. Org. Khim., 16, 450 (1980).
A. Maccoll, J. Chem. Soc., 670 (1946).
V. M. Dziomko, B. K. Berestevich, and R. S. Kuzanyan, Khim. Geterotsikl. Soedin., No. 6, 802 (1979).
V. M. Dziomko, B. K. Berestevich, A. V. Kessenikh, V. A. Olikova, and R. S. Kuzanyan, Khim. Geterotsikl. Soedin., No. 11, 1530 (1980).
French Patent No. 1403372; Chem. Abstr., 63, 14871c (1965).
I. I. Grandberg, Dinh Vei-Py, and A. N. Kost, Zh. Obshch. Khim., 31, 2311 (1961).
V. M. Dziomko, B. K. Berestevich, A. V. Kessenikh, R. S. Kuzanyan, and V. A. Olikova, NIITEKhIM Deposited Paper No. 305 khp-D80 (March 28, 1980).
A. Michaelis, Lieb. Ann., 339, 117 (1905).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 11, pp. 1524–1529, November, 1980.
Rights and permissions
About this article
Cite this article
Dziomko, V.M., Berestevich, B.K., Kessenikh, A.V. et al. Some side reactions in the template synthesis of macrocyclic compounds from o-amino-o'-haloazopyrazoles. Chem Heterocycl Compd 16, 1160–1165 (1980). https://doi.org/10.1007/BF00504115
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00504115