Abstract
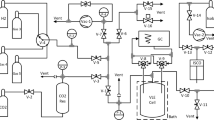
The fugacity coefficients of hydrogen in binary mixtures with methane and propane were measured using a physical equilibrium technique. This technique involves the use of an experimental chamber which is divided into two regions by a semipermeable membrane. Hydrogen can penetrate and pass through the membrane, while the other component (in this case, methane or propane) cannot. At equilibrium, pure hydrogen will permeate into one “compartment” of the chamber, while the binary mixture occupies the other compartment. Thus, the pressure of pure hydrogen on one side approaches the partial pressure of hydrogen in the mixture on the other side of the membrane. This allows a direct measurement of the hydrogen component fugacity at a given mixture mole fraction. In this study, results are reported for measurements made on the hydrogen+propane binary at 80°C (353 K) and 130°C (403 K) and the hydrogen+methane binary at 80°C (353 K). All measurements were performed with a total mixture pressure of 3.45 MPa. The experimental results are compared with predictions from the Redlich-Kwong, Peng-Robinson, and extended corresponding-states models.
Similar content being viewed by others
References
G. N. Lewis, Proc. Am. Acad. 37:49 (1901).
G. N. Lewis, Z. Phys. Chem. 38:205 (1901).
G. N. Lewis and M. Randall, Thermodynamics (McGraw-Hill, New York, 1961).
S. I. Sandler, Chemical and Engineering Thermodynamics (John Wiley & Sons, New York, 1977).
B. G. Kyle, Chemical and Process Thermodynamics (Prentice Hall, Englewood Cliffs, N.J., 1984).
J. M. Prausnitz, Molecular Thermodynamics of Fluid Phase Equilibria (Prentice Hall, Englewood Cliffs, N.J., 1969).
K. Denbigh, The Principles of Chemical Equilibrium (Cambridge University Press, New York, 1971).
J. M. Prausnitz, AIChE J. 5:3 (1969).
H. Y. Cheh, Proceedings of the 6th Symposium on Thermophysical Properties, P. E. Liley, ed. (Am. Soc. Mech. Eng., New York, 1973), p. 256.
D. P. Smith, Hydrogen in Metals (University of Chicago Press, Chicago, 1948).
E. M. Wise, Palladium (Academic Press, New York, 1968).
T. J. Bruno, J. Res. Natl. Bur. Std. 90(2):127 (1985).
T. J. Bruno, J. Chromatogr. Sci. 23(7):325 (1985).
T. J. Bruno and G. L. Hume, J. Res. Natl. Bur. Std. 90(3):225 (1985).
ASME Boiler and Pressure Vessel Code, Sect. VIII: Unfired Pressure Vessels (American Society of Mechanical Engineers, New York, 1965).
R. L. Powell, W. J. Hall, C. H. Hyink, L. L. Sparks, G. W. Burns, M. G. Scroger, and H. H. Plumb, Thermocouple Reference Tables Based on the IPTS-68, National Bureau of Standards, Monograph 125 (1975).
T. J. Bruno, D. E. Martire, M. W. P. Harbison, A. Nikolić, and C. F. Hammer, J. Phys. Chem. 87:2425 (1983).
E. Heftmann, Chromatography: A Laboratory Handbook of Chromatographic and Electrophoretic Methods, 3rd ed. (Van Nostrand Reinhold, New York, 1975).
H. M. McNair and E. J. Bonnelli, Basic Gas Chromatography (Varian Aerograph, 1969).
C. J. Cowper and A. J. DeRose, The Analysis of Gases by Chromatography (Pergamon Press, Oxford, 1983).
P. G. Jeffery and P. J. Kiping, Gas Analysis by Gas Chromatography (Pergamon Press, Oxford, 1972).
D.-Y. Peng and D. B. Robinson, Ind. Eng. Chem. Fund. 15:59 (1976).
R. T. Jacobsen and R. J. Stewart, J. Phys. Chem. Ref. Data 2:757 (1973).
F. Antezana and H. Y. Cheh, Ind. Eng. Chem. Fund. 14:224 (1975).
T. J. Bruno and G. L. Hume, Int. J. Thermophys. 7:1053 (1986).
R. C. Reid, J. M. Prausnitz, and T. K. Sherwood, The Properties of Gases and Liquids (McGraw-Hill, New York, 1976), p. 630.
R. A. Mentzer, R. A. Greenkorn, and K.-C. Chao, Ind. Eng. Chem. Process Des. Dev. 20:240 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bruno, T.J., Hume, G.L. & Ely, J.F. Hydrogen component fugacities in binary mixtures with methane and propane. Int J Thermophys 7, 1033–1051 (1986). https://doi.org/10.1007/BF00502376
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00502376