Summary
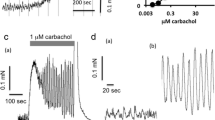
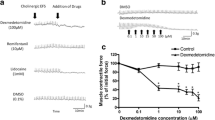
Isolated tracheal and bronchial strip-chain preparations of the rat were used to study the effect of temperature on electrically or acetylcholine-induced contraction. The preparations were suspended in the organ bath containing Krebs bicarbonate solution for isometric tension recording. A decrease of bath temperature from 37°C to 20°C (cooling) had no effect on basal tone but augmented the contractile responses of the trachea and bronchus caused by stimulation of intramural cholinergic nerves (0.5–5 Hz) or acetylcholine (3 μmol/1–0.3 mmol/l). Cooling-induced augmentation of the contractile response to acetylcholine was not affected by pretreatment of the tissue with physostigmine (0.1 μmol/l) or tetrodotoxin (0.3 μmol/l). The affinity of acetylcholine for the tracheal muscarinic receptors at 20°C, determined from its dissociation constant (K A), was not significantly different from that at 37°C. On the other hand, acetylcholine-induced contraction of trachea which was incubated with isosmotic K+-rich Krebs solution and with Ca-free, EGTA (0.1 mmol/l) containing Krebs solution were both augmented at 20°C. Caffeine or vanadate, each at a lower concentration than the threshold for causing contraction by itself, augmented the contractile responses of the trachea to acetylcholine (1 μmol/l–0.3 mmol/l). These potentiating effects of caffeine and vanadate were greater at 20°C then 37°C. From these observations, it is concluded that increased responsiveness of the rat airway smooth muscle to acetylcholine with lowered temperature may involve the acceleration of Ca release from intracellular storage sites, inhibition of Ca extrusion from the cell and or the inhibition of Ca reuptake by intracellular storage sites.
Similar content being viewed by others
References
Arunlakshana O, Schild HO (1959) Some quantitative uses of drug antagonists. Br J Pharmacol 14:48–58
Bauer H, Goodford PJ, Hüter J (1965) The calcium content and 45calcium uptake of the smooth muscle of the guinea-pig taenia coli. J Physiol 176:163–179
Bolton TB (1979) Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev 59:606–717
Coburn RF (1979) Electromechanical coupling in canine trachealis muscle: acetylcholine contraction. Am J Physiol 236:C177-C184
Coburn RF, Tomita T (1973) Evidence for nonadrenergic inhibitory nerves in the guinea pig trachealis muscle. Am J Physiol 224:1072–1080
Deal EC Jr, McFadden ER Jr, Ingram RH Jr, Jaeger JJ (1979a) Esophageal temperature during exercise in asthmatic and nonasthmatic subjects. J Appl Physiol: Respirat Environ Exercise Physiol 46:484–490
Deal EC Jr, McFadden ER Jr, Ingram RH Jr, Strauss RH, Jaeger JJ (1979b) Role of respiratory heat exchange in production of exercise-induced asthma. J Appl Physiol: Respirat Environ Exercise Physiol 46:467–475
DiPolo R, Rojas HR, Beaugé (1979) Vanadate inhibits uncoupled Ca efflux but not Na-Ca exchange in squid axons. Nature 281:228–229
Dupont Y, Bennett N (1982) Vanadate inhibition of the Ca2+-dependent conformational change, of the sarcoplasmic reticulum Ca2+-ATPase. FEBS Letters 139:237–240
Endo M (1977) Calcium release from the sarcoplasmic reticulum. Physiol Rev 57:71–108
Endo M, Yagi S, Iino M (1982) Tension-pCa relation and sarcoplasmic reticulum responses in chemically skinned smooth muscle fibers. Fed Proc 41:2245–2250
Foster RW, Small RC, Weston AH (1983) Evidence that the spasmogenic action of tetraethylammonium in guinea-pig trachealis is both direct and dependent on the cellular influx of calcium ion. Br J Pharmacol 79:255–263
Furchgott RF (1966) The use of β-haloalkylamines in the differentiation of receptors and in the determination of dissociation constants of receptor-agonist complexes. Adv Drug Res 3:21–55
Furchgott RE, Bursztyn P (1967) Comparison of dissociation constants and of relative efficacies of selected agonists acting of parasympathetic receptors. Ann NY Acad Sci 144:882–898
Hendrickx H, Casteels R (1974) Electrogenic sodium pump in arterial smooth muscle cells. Pflügers Arch 346:299–306
Iino M (1981) Tension responses of chemically skinned fibre bundles of the guinea-pig taenia caeci under varied ionic environments. J Physiol 320:449–467
Ishii T, Shimo Y (1979) Potassium-induced relaxations of the guinea-pig taenia coli. Arch Int Pharmacodyn Ther 239:36–44
Ishii T, Shimo Y (1980) Potassium-inducd relaxation of the rat anococcygeus muscle. Arch Int Pharmacodyn Ther 243:27–36
Ishii T, Shimo Y (1983) Effect of temperature on acetylcholineinduced contraction of isolated rat, tracheal muscle. Jpn J Pharmacol 33:179P
Ishii T, Shimo Y (1984) Inhibitory effects of procaine on the contractile responses of the guinea-pig taenia caecum to acetylcholine, substance P and potassium chloride. Naunyn-Schmiedeberg's Arch Pharmacol 326:175–180
Kamikawa Y, Shimo Y (1976) Pharmacological differences of non-adrenergic inhibitory response and of ATP-induced relaxation in guinea-pig tracheal strip-chains. J Pharm Pharmacol 28:854–855
Kirkpatrick (1981) Tracheobronchial smooth muscle. In: Bülbring E, Brading AF, Jones AW, Tomita T (ed) Smooth muscle: an assessment of current knowledge. Edward Arnold Publishers, London, pp 385–395
Lahrtz HG, Lüllmann H, Van Zwieten PA (1967) Calcium transport in isolated guinea-pig atria during metabolic inhibition. Biochim Biophys Acta 135:701–709
Murlas C, Tencati J, Mahutte K, Nadel JA, Roberts JM (1982) Direct effects of cooling on human bronchial smooth muscle. Am Rev Respir Dis 125:222
Nayler RA, Sparrow MP (1983) Mechanism of vanadate-induced contraction of airways smooth muscle of the guinea-pig. Br J Pharmacol 80:163–172
O'Neal SG, Rhoads DB, Racker E (1979) Vanadate inhibition of sarcoplasmic reticulum Ca2+-ATPase and other ATPases. Biochem Biophys Res Commun 89:845–850
Saida K (1981) Ca2+- and ‘depolarization’-induced Ca2+ release in skinned smooth muscle fibers. Biomedical Res 2:453–455
Saida K (1982) Intracellular Ca release in skinned smooth muscle. J Gen Physiol 80:191–202
Sakai T, Geffner ES, Sandow A (1971) Caffeine contracture in muscle with disrupted transverse tubules. Am J Physiol 220:712–717
Sakai T, Kurihara S (1974) A study on rapid cooling contracture from the viewpoint of excitation-contraction coupling. Jikei Med J 21:47–88
Somlyo AP, Devine CE, Somlyo AV, North SR (1971) Sarcoplasmic reticulum and the temperature-dependent contraction of smooth muscle in calcium-free solutions. J Cell Biol 51:722–741
Souhrada M, Souhrada JF (1981) The direct effect of temperature on airway smooth muscle. Respir Physiol 44:311–323
Souhrada, M, Souhrada JF, Cherniack RM (1981) Evidence for a sodium electrogenic pump in airway smooth muscle. J Appl Physiol: Respirat Environ Exercise Physiol 51:346–352
Strauss RH, McFadden ER Jr, Ingram RH, Jr, Deal EC Jr, Jaeger JJ (1978) Influence of heat and humidity on the airway obstruction induced by exercise in asthma. J Clin Invest 61:433–440
Strauss RH, McFadden ER Jr, Ingram RH Jr, Jaeger JJ (1977) Enhancement of exercise-induced asthma by cold air. N Engl J Med 297:743–747
Van Breemen C, Aaronson P, Loutzenhiser R (1979) Sodiumcalcium interactions in mammalian smooth muscle. Pharmacol Rev 30:167–208
Zeballos RJ, Shturman-Ellstein R, McNally JF Jr, Hirsch JE, Souhrada JF (1978) The role of hyperventilation in exerciseinduced bronchoconstriction. Am Rev Respir Dis 118:877–884
Author information
Authors and Affiliations
Additional information
This work was supported in part by Scientific Research Fund 58770177, from the Ministry of Education, Science and Culture, Japan
Rights and permissions
About this article
Cite this article
Ishii, T., Shimo, Y. Cooling-induced supersensitivity to acetylcholine in the isolated airway smooth muscle of the rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 329, 167–175 (1985). https://doi.org/10.1007/BF00501208
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00501208