Summary
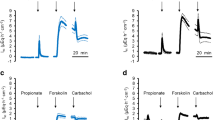
The release of cyclic adenosine 3′:5′-monophosphate (cAMP) from guinea-pig and rabbit gallbladder was investigated in vitro. Serosal addition of prostaglandin E1 (PGE1) to luminally perfused guinea-pig gallbladders caused a concentration-dependent efflux of cAMP to the mucosal side, the threshold concentration of PGE1 being 10−7 M. The efflux of cAMP to the serosal side was 7-fold lower. A mucosal sidedness of cAMP release was also observed in stripped preparations of rabbit gallbladder mucosa mounted between two half chambers. No cAMP was found in the solutions bathing the serosal layers isolated from rabbit gallbladders. Fluid secretion was observed at 10−7 M PGE1, an effect mimicked by serosal, but not mucosal application of cAMP (3.3×10−3 M). This is taken to indicate that the basolateral membrane is more easily permeated by cAMP than the apical membrane, since cAMP is believed to exerts its physiological effects from inside the cell. It is concluded that preferential release of cAMP to the mucosal side is not due to a higher permeability of the brush border membrane but rather represents an as yet undefined transport process which may be of importance for the regulation of excessive intracellular cAMP levels.
Similar content being viewed by others
References
Andersson KE, Andersson R, Hedner P, Persson CGA (1973) Parallelism between mechanical and metabolic responses to cholecystokinin and prostaglandin E2 in extrahepatic biliary tract. Acta Physiol Scand 89:571–579
Araki N, Nagata N, Kimura N (1977) Effects of parathyroid hormone and prostaglandin E1 in vitro on release of cyclic AMP from kidney cortical tissue. Endocrinol Jpn 24:581–587
Barber R, Butcher RW (1981) The quantitative relationship between intracellular concentration and egress of cyclic AMP from cultured cells. Mol Pharmacol 19:38–43
Butlen D, Jard S (1972) Renal handling of 3′–5′-cyclic AMP in the rat. The possible role of luminal 3′–5′-cyclic AMP in the tubular reabsorption of phosphate. Pflügers Arch 331:172–190
Diamond JM (1962) The reabsorptive function of the gallbladder. J Physiol (London) 161:442–473
Doore BJ, Bashor MM, Spitzer N, Mawe RC, Saier MH (1975) Regulation of adenosine 3′:5′-monophosphate efflux from rat glioma cells in culture. J Biol Chem 250:4371–4372
Frömter E, Diamond JM (1972) Route of passive ion movement in epithelia. Nature New Biol 235:9–13
Fülgraff G, Brandenbusch G, Heintze K, Meiforth A (1975) Renal effects of the prostaglandins A1 and E2 in hydrated and hydropenic dogs. Experientia 31:65–66
Hawlina A, Osswald H (1979) Cyclic nucleotides in renal tissue and urine during graded expansion of extracellular fluid volume in intact and acutely parathyroidectomized rats. Res Exp Med (Berl) 175:139–148
Heintze K, Leinesser W, Petersen KU, Heidenreich O (1975) Triphasic effect of prostaglandins E1, E2 and F2α on the fluid transport of isolated gallbladder of guinea-pigs. Prostaglandins 9:309–322
Heintze K, Petersen KU, Olles P, Saverymuttu SH, Wood JR (1979) Effects of bicarbonate on fluid and electrolyte transport by the guinea-pig gallbladder: A bicarbonate-chloride exchange. J Membr Biol 45:43–59
Kaminsky NI, Broadus AE, Hardman IG, Douglas JJ, Ball JH, Sutherland EW, Liddle GW (1970) Effects of parathyroid hormone on plasma and urinary adenosine 3′,5′-monophosphate in man. J Clin Invest 49:2387–2395
King CD, Mayer SE (1974) Inhibition of egress of adenosine 3′,5′-monophosphate from pigeon erythrocytes. Mol Pharmacol 100:941–953
Lauterbach F (1977) Passive permeabilities of luminal and basolateral membranes in the isolated mucosal epithelium of guinea-pig small intestine. Naunyn-Schmiedeberg's Arch Pharmacol 297:201–212
Lowry OH, Rosebrough NF, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–271
Osswald H, Hawlina A (1979) Effects of acetazolamide and changes of acid-base balance on the content of cyclic nucleotides in the rat kidney. Pharmacology 19:44–50
Petersen KU, Holme M (1980) Asymmetric release of cAMP from rabbit isolated colonic mucosa. Effects of ouabain, Na-free solution, loop diuretics and morphine. Naunyn-Schmiedeberg's Arch Pharmacol 311:R1
Petersen KU, Busch L, Sturm KW, Osswald H, Heintze K (1976) Characterisation of the prostaglandin-induced inhibition of fluid transport in the isolated gallblader of guinea-pigs. In: Samuelsson B, Paoletti R (eds) Advances in prostaglandins and thromboxane research, Vol 2. Raven Press, New York, pp 941–942
Plagemann PGW, Erbe J (1977) Exit transport of a cyclic nucleotide from mouse l-cells. J Biol Chem 252:2010–2016
Plagemann PGW, Wohlhueter RM (1980) Permeation of nucleosides, nucleic acid bases, and nucleotides in animal cells. In: Bronner F, Kleinzeller A (eds) Current topics in membranes and transport, Vol 14. Academic Press, New York London Toronto Sidney San Francisco, pp 226–330
Rindler MJ, Bashor MM, Spitzer N, Saier MH (1978) Regulation of adenosine 3′:5′-monophosphate efflux from animal cells. J Biol Chem 253:5431–5436
Saier MH, Feucht BU, McCaman MT (1975) Regulation of intracellular adenosine 3′:5′-monophosphate levels in Escherischia coli and Salmonella typhimurium. J Biol Chem 250:7593–7601
Selstam G, Janson PO, Eden S (1976) Effect of LH on the release of cyclic AMP by the rabbit ovary perfused in vivo and in vitro. J Reprod Fertil 46:355–358
Steinberg RA, Steinberg MG, Wetters TVD (1979) A variant of S 49 mouse lymphoma cells with enhanced secretion of cyclic AMP. J Cell Physiol 100:579–588
Sutton RAL, Quamme GA, Dirks JH (1979) Transport of calcium, magnesium and inorganic phosphate in the kidney. In: Giebisch G, Tosteson DC, Ussing HH (eds) Membrane transport in biology, vol IVa. Springer, Berlin Heidelberg New York, pp 357–412
Tonoue T, Kitoh J (1978) Release of cyclic AMP from the chicken thyroid stimulated with TSH in vitro. Endocrinol Jpn 25:105–109
Urakabe S, Handler JS, Orloff J (1975) Release of cyclic AMP by toad urinary bladder. Am J Physiol 228:954–958
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Petersen, KU., Osswald, H. & Heintze, K. Asymmetric release of cyclic AMP from guinea-pig and rabbit gallbladder. Naunyn-Schmiedeberg's Arch. Pharmacol. 318, 358–362 (1982). https://doi.org/10.1007/BF00501178
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00501178