Summary
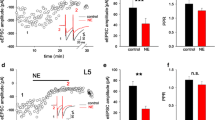
Pressure ejection of l-norepinephrine (NE) in the in vivo rat hippocampus generally produced depression of pyramidal cell spontaneous activity. In addition, both excitation and biphasic responses were observed. NE-induced inhibition of firing rate was effectively antagonized by concurrent administration of the alpha antagonist phentolamine, but was largely unaltered by the beta antagonist timolol. On the other hand, NE-induced elevation in spontaneous firing rate was effectively blocked by timolol, and largely unaffected by phentolamine. Another beta antagonist, sotalol, did not selectively antagonize either NE-induced inhibition or NE-induced excitation. The beta agonist 2-fluoro-NE produced increases in pyramidal cell firing rates in most cells studied, while the alpha agonist 6-fluoro-NE inhibited the majority of cells examined. The effects of sotalol were also examined on alpha and beta receptor-mediated field responses in the in vitro hippocampal slice. Sotalol was shown to be a selective beta antagonist in this system, blocking excitation evoked by the beta agonist isoproterenol while having no effect on inhibition elicited by the alpha agonist clonidine; however, the potency of sotalol (K i=3.5μM) was considerably less than that of timolol (K i=50 nM). Taken together, these results suggest that NE-induced depression and elevation in hippocampal pyramidal cell spontaneous discharge in vivo are mediated via alpha and beta adrenoceptors, respectively.
Similar content being viewed by others
References
Andén NE, Dahlström A, Fuxe K, Larsson K, Olson L, Ungerstedt U (1966) Ascending monoamine neurons to the telencephalon and diencephalon. Acta Physiol Scand 67:313–325
Andersen P (1960) Interhippocampal impulses. II. Apical dendritic activation of CA1 neurons. Acta Physiol Scand 48:178–208
Andersen P, Bliss TVP, Skrede KK (1971) Unit analysis of the hippocampal population spike. Exp Brain Res 13:208–221
Andersen P, Holmqvist E, Voorhoeve PE (1966) Entorhinal activation of dentate granule cells. Acta Physiol Scand 66:448–460
Biscoc TJ, Straughan DW (1966) Micro-electrophoretic studies of neurons in the rat hippocampus. J Physiol (Lond) 183:341–359
Blackstad TW, Fuxe K, Hökfelt T (1967) Noradrenaline nerve terminals in the hippocampal region of the rat and the guinea pig. Z Zellforsch 7:463–473
Clark BJ (1976) Pharmacology of the beta-adrenoceptor blocking agents. In: Saxena PR, Forsyth RP (eds) Beta-adrenoceptor blocking drugs. North-Holland Press, Amsterdam, pp 45–76
Crutcher KA, Davis JN (1980) Hippocampal alpha- and beta-adrenergic receptors: Comparisons of [3H] dihydroalprenol and [3H] WB 4101 binding with noradrenergic innervation in the rat. Brain Res 182:107–117
Daly JW, Padgett W, Nimitkitpaisan Y, Creveling CR, Cantacuzene D, Kirk KL (1980) Specific agonists for the activation of alpha and beta adrenergic-sensitive cyclic AMP-generating systems in brain slices. J Pharmacol Exp Ther 212:382–389
Dunwiddie T, Lynch G (1978) Long-term potentiation and depression of synaptic responses in the rat hippocampus: Localization and frequency dependency. J Physiol (Lond) 276:353–367
Dunwiddie T, Mueller A, Palmer M, Stewart J, Hoffer B (1980) Electrophysiological interactions of enkephalins with neuronal circuitry in the rat hippocampus. I. Effects on pyramidal cell activity. Brain Res 184:311–330
Freedman R, Hoffer BJ, Woodward DJ (1975) A quantitative microiontophoretic analysis of the responses of central neurons to noradrenaline. Br J Pharmacol 54:529–531
Freedman R, Hoffer BJ, Woodward DJ, Puro D (1977) Interaction of norepinephrine with cerebellar activity evoked by mossy and climbing fibers. Exp Neurol 55:269–288
Fujita Y, Sakata H (1962) Electrophysiological properties of CA1 and CA2 apical dendrites of rabbit hippocampus. J Neurophysiol 25:209–222
Fuxe K (1965) Evidence for the existence of monoamine neurons in the central nervos system. IV. The distribution of monoamine terminals in the central nervous system. Acta Physiol Scand (Suppl) 247:37–85
Geller HM, Woodward DJ (1972) An improved constant current source for microiontophoretic drug application studies. Electroencephalogr Clin Neurophysiol 33:430–433
Herz A, Nacimiento AC (1965) Über die Wirkung von Pharmaka auf Neurone des Hippocampus nach microelectrophoretischer Verabfolgung. Naunyn-Schmiedebergs Arch Exp Path Pharmak 251:295–314
Hoffer BJ, Neff NH, Siggins GR (1971) Microiontophoretic release of norepinephrine from micropipettes. Neuropharmacology 20:175–180
Hoffer BJ, Siggins GR, Bloom FE (1971) Studies on norepinephrinecontaining afferents to Purkinje cells of rat cerebellum. II. Sensitivity of Purkinje cells to norepinephrine and related substances administered by microiontophoresis. Brain Res 25:523–534
Johnson ES, Roberts MHT, Straughan DW (1969) The responses of cortical neurons to monoamines under differing anaesthetic conditions. J Physiol (Lond) 203:261–280
Jones BE, Moore RY (1977) Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res 127:23–53
Jordan LM, Lake N, Phillis JW (1972) Mechanism of noradrenaline depression of cortical neurones: A species comparison. Eur J Pharmacol 20:381–384
Krnjević K, Phillis JW (1963) Actions of certain amines on cerebral cortical neurones. Br J Pharmacol 20:471–490
Lake N, Jordan LM, Phillis JW (1972) Mechanism of noradrenaline action in cat cerebral cortex. Nature New Biol 240:249–250
Lee HK, Dunwiddie T, Hoffer B (1980) Electrophysiological interactions of enkephalins with neuronal circuitry in the rat hippocampus. II. Effects on interneuron excitability. Brain Res 184:331–342
Lindvall O, Björklund A (1974) The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand (Suppl) 412:1–48
Lømo T (1971) Patterns of activation in a monosynaptic cortical pathway: The perforant path input to the dentate area of the hippocampal formation. Exp Brain Res 12:18–45
Marwaha J, Palmer M, Hoffer B, Freedman R (1980a) Phencyclidineinduced depressions of cerebellar Purkinje neurons. Life Sci 26:1509–1515
Marwaha J, Palmer MR, Woodward DJ, Hoffer BJ, Freedman R (1980b) Electrophysiological evidence for presynaptic actions of phencyclidine on noradrenergic terminals in rat cerebellum. J Pharmacol Exp Ther 215:606–613
McCaman RE, McKenna DG, Ono JK (1977) A pressure ejection system for intracellular and extracellular ejections of picoliter volumes. Brain Res 136:141–147
Moises HC, Woodward DJ, Hoffer BJ, Freedman R (1979) Interactions of norepinephrine with Purkinje cell responses to putative amino acid neurotransmitters applied by microiontophoresis. Exp Neurol 64:493–515
Mueller AL, Hoffer BJ, Dunwiddie TV (1981) Noradrenergic responses in rat hippocampus: Evidence for mediation by alpha and beta receptors in the in vitro slice. Brain Res 214:113–126
Palacios JM, DeHaven RN, Kuhar MJ (1980) Localization of betaadrenergic receptors in rat brain by light microscopic autoradiography. Fed Proc 39:593
Palmer MR, Wuerthele SM, Hoffer BJ (1980) Physical and physiological characteristics of micropressure ejection of drugs from multibarreled pipettes. Neuropharmacology 19:931–938
Sakai M, Swartz BE, Woody CD (1979) Controlled micro-release of pharmacological agents: Measurements of volume ejected in vitro through five-tipped glass microelectrodes by pressure. Neuropharmacology 18:209–213
Salmoiraghi GC, Stefanis CN (1965) Patterns of central neurons responses to suspected transmitters. Arch Ital Biol 103:705–724
Segal M, Bloom FE (1974a) The action of norepinephrine in the rat hippocampus. I. Iontophoretic studies. Brain Res 72:79–97
Segal M, Bloom FE (1974b) The action of norepinephrine in the rat hippocampus. II. Activation of the input pathway. Brain Res 72:99–114
Segal M, Pickel V, Bloom FE (1973) The projections of the nucleus locus coeruleus: An autoradiographic study. Life Sci 13:817–821
Smith BM, Hoffer BJ (1978) A gated high voltage iontophoresis system with accurate current monitoring. Electroencephalogr Clin Neurophysiol 44:398–402
Sorensen S, Palmer M, Dunwiddie T, Hoffer B (1980) Electrophysiological correlates of ethanol-induced sedation in differentially sensitive lines of mice. Science 210:1143–1145
Stefanis C (1964) Hippocampal neurons: Their responsiveness to microelectrophoretically administered endogenous amines. Pharmacologist 6:171
Stone TW (1973) Pharmacology of pyramidal tract cells in the cerebral cortex: Noradrenaline and related substances. Naunyn-Schmiedeberg's Arch Pharmacol 278:333–346
Storm-Mathisen J (1977) Localization of transmitter candidates in the brain: The hippocampal formation as a model. Prog Neurobiol 8:119–181
Szabadi E (1979) Adrenoceptors on central neurons. Microelectrophoretic studies. Neuropharmacology 18:831–843
Szabadi E, Bradshaw CM, Bevan P (1977) Excitatory and depressant neuronal responses to noradrenaline, 5-hydroxytryptamine and mescaline: The role of the baseline firing rate. Brain Res 126:580–583
Ungerstedt U (1971) Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand (Suppl) 367:1–48
Waterhouse BD, Moises HC, Woodward DJ (1979) Alpha, beta pharmacological characterization of noradrenergic modulatory actions in rat somatosensory cortex. Soc Neurosci (Abstr) 6:356
Wikberg JES (1979) The pharmacological classification of adrenergic alpha-1 and alpha-2 receptors and their mechanisms of action. Acta Physiol Scand (Suppl) 468:1–99
Woodward DJ, Moises HC, Waterhouse BD, Hoffer BJ, Freedman R (1979) Modulatory actions of norepinephrine in the central nervous system. Fed Proc 38:2109–2116
Young WS, Kuhar MJ (1980) Noradrenergic alpha-1 and alpha-2 receptors: Light microscopic autoradiographic localization. Fed Prod 39:593
Zieglgänsberger W, French ED, Siggins GR, Bloom FE (1979) Opioid peptides may excite hippocampal pyramidal neurons by inhibiting adjacent inhibitory interneurons. Science 205:415–417
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mueller, A.L., Palmer, M.R., Hoffer, B.J. et al. Hippocampal noradrenergic responses in vivo and in vitro. Naunyn-Schmiedeberg's Arch. Pharmacol. 318, 259–266 (1982). https://doi.org/10.1007/BF00501163
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00501163