Summary
-
1.
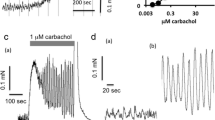
In the isolated perfused rabbit ear bradykinin (B) and acetylcholine (ACh) stimulate paravascular pain receptors. Changed calcium (Ca2+) and potassium (K+) concentrations were used to investigate whether there were different “pharmacological” receptors for B and ACh.
-
2.
Increased [K+] (26.8 mM) lowered the pain threshold for both B and ACh. Decreased [K+] (0.27 mM) did not alter the algesic effect of B or ACh.
-
3.
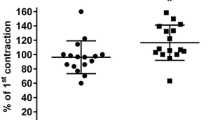
Increased [Ca2+] (3.6 and 9.0 mM) strongly enhanced the algesic effect of ACh but reduced that of B. Decreased [Ca2+] (0.18 and 0.6 mM) also reduced the algesic effect of B but did not influence that of ACh. Single i.a. injections of CaCl2 (1–5 mg) directly stimulated paravascular pain receptors.
-
4.
Prostaglandin E1 (PGE1, 10 ng/ml) reversed the inhibitory influence of 9 mM [Ca2+] on the B effect and further enhanced the Ca2+-facilitated ACh effect.
-
5.
Conclusion. ACh and B exert their algesic effect via different receptor sites. This was concluded from the use of changed [Ca2+] and [K+]. High [K+] increased the effect of B and ACh due to membrane depolarization. Low [Ca2+] diminished only the effect of B but not that of ACh. High [Ca2+] increased the algesic effect of ACh possibly due to an enhanced Ca2+ influx at the generator region thus contributing to excitation. This finding is substantiated by the fact that Ca2+ by itself in sufficiently high amounts can stimulate pain receptors. High [Ca2+] reduced the effect of B by interfering with the pain enhancing action of PGE released by B, the algesic effect of which being strongly dependent on the presence of PGE.
An interference of high [Ca2+] with the direct effect of B is rather unlikely since during inhibited PGE-release high [Ca2+] no longer reduces significantly the effect of B.
Similar content being viewed by others
References
Benjamin, F. B.: Release of intracellular potassium as the physiological stimulus for pain. Fed. Proc. 18, 10 (1959)
Brink, F., Bronk, D. W., Larrabee, M. G.: Chemical excitation of nerve. Ann. N. Y. Acad. Sci. 47, 457–485 (1946)
Douglas, W. W., Poisner, A. M.: On the mode of action of acetylcholine in evoking adrenal medullary secretion: Increased uptake of calcium during the secretory response. J. Physiol. (Lond.) 162, 385–392 (1962)
Eagling, E. M., Lovell, H. G., Pickles, V. R.: Interaction of prostaglandin E1 and calcium in the guinea-pig myometrium. Br. J. Pharmacol. 44, 510–516 (1972)
Ferreira, S. H., Moncada, S., Vane, J. R.: Prostaglandins and the mechanism of analgesia produced by aspirin-like drugs. Br. J. Pharmacol. 49, 86–97 (1973)
Ferreira, S. H., Moncada, S., Vane, J. R.: Potentiation by prostaglandins of the nociceptive activity of bradykinin in the dog knee joint. Br. J. Pharmacol. 50, 461P (1974)
Ginzel, K. H.: The importance of sensory nerve endings as sites of drug action. Naunyn-Schmiedeberg's Arch. Pharmacol. 288, 29–56 (1975)
Hadházy, P., Todorov, S., Nador, T.: Antagonism by Ca2+ of the inhibitory action of PGE1 on the electrically induced vasoconstriction in the rabbit ear artery. Eur. J. Pharmacol. 34, 393–395 (1975)
Hedqvist, P.: Antagonism by calcium of the inhibitory action of prostaglandin E2 on sympathetic neurotransmission in the cat spleen. Acta Physiol. Scand. 80, 269–275 (1970)
Johnson, D. G., Thoa, N. B., Weinshilboum, R., Axelrod, J., Kopin, I. J.: Enhanced release of dopamine β-hydroxylase from sympathetic nerves by calcium and phenoxybenzamine and its reversal by prostaglandins. Proc. Natl. Acad. Sci. U.S.A. 68, 2227–2230 (1971)
Juan, H., Lembeck, F.: Influence of prostaglandin E1, indomethacin, calcium and potassium on the action of nociceptive substances. Naunyn-Schmiedeberg's Arch. Pharmacol. 282, R42 (1974a)
Juan, H., Lembeck, F.: Action of peptides and other algesic agents on paravascular pain receptors of the isolated perfused rabbit ear. Naunyn-Schmiedeberg's Arch. Pharmacol. 283, 151–164 (1974b)
Juan, H., Lembeck, F.: Inhibition of the action of bradykinin and acetylcholine on paravascular pain receptors by tetrodotoxin and procaine. Naunyn-Schmiedeberg's Arch. Pharmacol. 290, 389–395 (1975)
Juan, H., Lembeck, F.: Release of prostaglandins from the isolated perfused rabbit ear by bradykinin and acetylcholine. Agents and Actions, 6, 642–645 (1976)
Kanno, M., Dunning, B. B., Machne, X.: Calcium dependence of acetylcholine induced conductance changes. Life Sci. 18, 311–318 (1976)
Lembeck, F.: Untersuchungen über die Auslösung afferenter Impulse. Naunyn-Schmiedebergs Arch. Exp. Path. Pharmak. 230, 1–9 (1957)
Lembeck, F., Juan, H.: Interaction of prostaglandins and indomethacin with algesic substances. Naunyn-Schmiedeberg's Arch. Pharmacol. 285, 301–313 (1974)
Lembeck, F., Popper, H., Juan, H.: Release of prostaglandins by bradykinin as an intrinsic mechanism of its algesic effect. Naunyn-Schmiedeberg's Arch. Pharmacol. 294, 69–73 (1976)
Lim, R. K. S.: A revised concept of the mechanism of analgesia and pain. In: R. S. Knighton and P. R. Dumke: Pain, pp. 117–154. Boston: Little Brown 1966
Magazanik, L. G., Vyskocil, F.: Dependence of acetylcholine desensitization on the membrane potential of frog muscle fibre and the ionic changes in the medium. J. Physiol. (Lond.) 210, 507–518 (1970)
McGiff, J. C., Terragno, N. A., Malik, K. U., Lonigro, A. J.: Release of prostaglandin E-like substance from canine kidney by bradykinin. Circ. Res. 31, 36–43 (1972)
Moncada, S., Ferreira, S. H., Vane, J. R.: Inhibition of prostaglandin biosynthesis as the mechanism of analgesia of aspirinlike drugs in the dog knee joint. Eur. J. Pharmacol. 31, 250–260 (1975)
Paintal, A. S.: Effect of drugs on vertebrate mechanoreceptors. Pharmacol. Rev. 16, 341–380 (1964)
Palmer, M. A., Piper, P. J., Vane, J. R.: Release of rabbit aorta contracting substance (RCS) and prostaglandins induced by chemical or mechanical stimulation of guinea-pig lungs. Br. J. Pharmacol. 49, 226–242 (1973)
Parsons, R. L., Cochrane, D. E., Schnitzler, R. M.: End-plate desensitization: specifity of calcium. Life Sci. 13, 459–465 (1973)
Schreiner, H. J.: Das Wärmegefühl nach Calciuminjektionen. Inaug-Diss., Göttingen 1936
Stjärne, L.: Kinetics of secretion of sympathetic neurotransmitter as a function of external calcium: mechanism of inhibitory effect of prostaglandin E. Acta Physiol. Scand. 87, 428–430 (1973)
Takeuchi, N.: Effects of calcium on the conductance change of the end-plate membrane during the action of transmitter. J. Physiol. (Lond.) 167, 141–155 (1963)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lembeck, F., Juan, H. Influence of changed calcium and potassium concentration on the algesic effect of bradykinin and acetylcholine. Naunyn-Schmiedeberg's Arch. Pharmacol. 299, 289–294 (1977). https://doi.org/10.1007/BF00500323
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00500323