Abstract
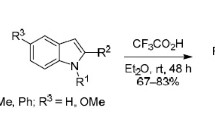
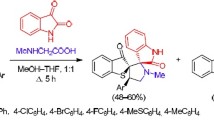
In the condensation of N-chloromethylisatin with indole and 2-methylindole in the presence of triethylamine, instead of the expected N-skatylisatins the products of their subsequent transformations with opening of the five-membered ring of isatin -o-(N-skatylamino)- and o[N-(2-methylskatyl)amino]benzoylcaproic acids —were isolated. In addition to the formation of these α-keto acids, under the indicated conditions one observes dimerization of N-chloromethylisatin and N-chloromethyl-5-methylisatin to give 2-(1-isatinylmethyloxy-3H-indolin-3-one and 2-(5-methyl-1-isatinylmethyloxy)-5-methyl-3H-indolin-3-one, respectively, i.e., dimers containing isatin rings in lactam and lactim forms. The structures of the compounds were confirmed by IR, PMR, and mass-spectral data.
Similar content being viewed by others
Literature cited
A. Kamal, M. Anjim, and S. Aziz Azadullah, Pak. J. Sci. Ind. Res., 9, 323 (1966); Chem. Abstr., 71, 3199 (1969).
A. Baeyer and S. Oekonmides, Ber., 15, 2093 (1882).
J. A. Barth, J. Prakt. Chem., 315, 339 (1973).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 2, pp. 217–220, February, 1977.
Rights and permissions
About this article
Cite this article
Zhungietu, G.I., Sinyavskaya, L.P. & Filipenko, T.Y. Reaction of 1-chloromethylisatin with indole. Chem Heterocycl Compd 13, 174–176 (1977). https://doi.org/10.1007/BF00497872
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00497872