Abstract
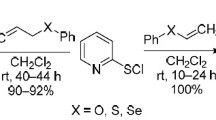
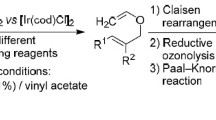
A number of 1-vinylpyrroles were obtained in up to 97% yields by base-catalyzed addition of substituted pyrroles to acetylene in dimethyl sulfoxide at 80–100°C.
Similar content being viewed by others
Literature cited
P. Waculik, The Chemistry of Monomers [Russian translation], Inostr. Lit., Moscow (1960).
M. F. Shostakovskii, G. G. Skvortsova, and E. S. Domnina, Usp. Khim., 38, 892 (1969).
B. A. Trofimov, A. I. Mikhaleva, G. A. Kalabin, and A. S. Atavin, Summaries of Papers Presented at the Fourth All-Union Colloquium on the Chemistry and Pharmacology of Indole Compounds [in Russian], Kishinev (1975), p. 24.
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 2, pp. 213–214, February, 1977.
Rights and permissions
About this article
Cite this article
Trofimov, B.A., Mikhaleva, A.I., Korostova, S.E. et al. Vinylation of pyrroles in dimethyl sulfoxide. Chem Heterocycl Compd 13, 170–171 (1977). https://doi.org/10.1007/BF00497870
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00497870