Summary
Cardiovascular alterations in hypo- and hyperthyroidism have been ascribed to changes of noradrenergic neurotransmission. In the present study the influence of thyroid hormones on adrenoceptors in the rat heart was further characterized. The effect of artificial hypothyroidism (induced by feeding 6-propyl-2-thiouracil, PTU) and hyperthyroidism (induced by daily injections of triiodothyronine, T3) on myocardial adrenoceptor binding, catecholamines, some physiological responses, and their interdependence was examined.
-
1.
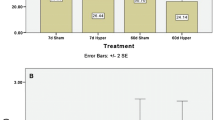
The density of myocardial β-adrenergic binding sites (3H-dihydroalprenolol, 3H-DHA) was reduced after PTU (by 38%) and enhanced after T3 treatment (by up to 82%). The increase was dose- and time-dependent and reversible within 4 days. No changes of the affinity of 3H-DHA to its binding sites were observed. Only L-T3 and L-T4 proved to be active, D-T3 and reverse T3 had no effect. The rise in β-adrenoceptor density caused by T3 was prevented by concomitant administration of cycloheximide, indicating its dependence on protein synthesis.
-
2.
The density of myocardial α 1-adrenergic binding sites (3H-prazosin) was significantly reduced in the PTU group (by up to 28%) and even more distinctly by T3 treatment (by up to 50%). K D values remained unaltered.
-
3.
The noradrenaline content and turnover of rat hearts was significantly reduced by T3-induced hyperthyroidism. PTU treatment had no influence on content and turnover of noradrenaline. Plasma noradrenaline as well as adrenaline levels in freely moving rats were increased by PTU treatment 9- and 5-fold, respectively. In T3-injected animals no significant changes were measured.
-
4.
The density of adrenoceptors is known to be inversely correlated with catecholamine levels in several organs. Neither α- nor β-adrenoceptor changes in the myocardium of dysthyroid rats could be attributed to such a homologous regulation, since they still occurred after chemical sympathectomy with 6-hydroxydopamine and adrenalectomy.
-
5.
Hypertrophy of the heart due to T3 could not be explained by prolonged β-adrenergic stimulation because it was not inhibited by 6-hydroxydopamine or high doses of propranolol. A T3-induced tachycardia was recorded in pithed and in intact rats. It was not reduced to normal levels by the β-adrenoceptor antagonist sotalol and, thus, was independent of sympathetic influence. Hypothyroid pithed rats displayed a marked bradycardia, whereas in intact hypothyroid animals a normal heart rate was measured at rest. Obviously, an enhanced availability of catecholamines which seems to reflect an increased release and/or a central nervous compensatory mechanism was responsible for the maintenance of the normal heart rate.
-
6.
In pithed rats the β-adrenoceptor-mediated increase in heart rate was attenuated by PTU treatment. The isoprenaline dose-response curve was shifted to the right, the maximal response was reduced. After T3 injections, the sensitivity to isoprenaline was not affected, but the maximal heart rate that could be obtained was increased. These results are compatible with the β-adrenoceptor changes described above.
It is concluded that cardiovascular signs of hypo- and hyperthyroidism can only be explained by a complex interaction of several factors. Beside the changes of adrenoceptor density and an altered sensitivity to noradrenaline, a central nervous regulation and subsequent changes of catecholamine release as well as effects independent of the sympathetic nervous system have to be considered.
Similar content being viewed by others
References
Axelrod J, Tomchick R (1958) Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem 233:702–705
Banerjee SP, Kung LS (1977) β-Adrenergic receptors in rat heart: effects of thyroidectomy. Eur J Pharmacol 43:207–208
Bayliss RIS, Edwards OM (1971) Urinary excretion of free catecholamines in Graves' disease. J Endocrinol 49:167–173
Bilezikian JP, Loeb JN (1983) The influence of hyperthyroidism and hypothyroidism on α- and β-adrenergic receptor systems and adrenergic responsiveness. Endocrine Rev 4:378–388
Brodde OE, Schümann HJ, Wagner J (1980) Decreased responsiveness of the adenylate cyclase system on left atria from hypothyroid rats. Mol Pharmacol 17:180–186
Brodie BB, Costa E, Dlabac A, Neff N, Smookler H (1966) Application of steady state kinetics to the estimation of synthesis rate and turnover time of tissue catecholamines. J Pharmacol Exp Ther 154:493–498
Bylund DB, U'Prichard DC (1983) Characterization of α 1- and α 2-adrenergic receptors. Int Rev Neurobiol 24:343–431
Bylund DB, Snyder SH (1976) Beta adrenergic receptor binding in membrane preparations from mammalian brain. Mol Pharmacol 12:568–580
Christensen NJ (1972) Increased levels of plasma noradrenaline in hypothyroidism. J Clin Endocrinol Metab 35:359–363
Christensen NJ (1973) Plasma noradrenaline and adrenaline in patients with thyrotoxicosis and myxedema. Clin Sci Mol Med 45:163–171
Ciaraldi T, Marinetti GV (1977) Thyroxine and propylthiouracil effects in vivo on alpha and beta adrenergic receptors in rat heart. Biochem Biophys Res Comm 74:984–991
Costa E (1970) Simple neuronal models to estimate turnover rate of noradrenergic transmitters in vivo. Biochem Psychopharmacol 2:169–204
Coulombe P, Dussault JH, Walker P (1976) Plasma catecholamine concentrations in hyperthyroidism and hypothyroidism. Metabolism 25:973–979
Da Prada M, Zürcher G (1976) Simultaneous radioenzymatic determination of plasma and tissue adrenaline, noradrenaline and dopamine within the femtomole range. Life Sci 19:1161–1174
Flavahan NA, McGrath JC (1982) α 1-Adrenoceptor activation can increase heart rate directly or decrease it indirectly through parasympathetic activation. Br J Pharmacol 77:319–328
Ghione S, Pellegrini M, Buzzigoli G, Carpi A, Valori C, Donato L (1974) Plasma and urinary catecholamine levels and thyroid activity in relation to cardiovascular changes in hyper- and hypothyroids. Horm Metab Res 6:93
Gillespie JS, McLaren A, Pollock D (1970) A method of stimulating different segments of the autonomic outflow from the spinal column to various organs in the pithed cat and rat. Br J Pharmacol 40:257–267
Greengrass P, Bremner R (1979) Binding characteristics of 3H-prazosin to rat brain α-adrenergic receptors. Eur J Pharmacol 55:323–326
Gross G (1982) Thyroid hormone dependent β-adrenoceptor changes in rat tissues. Naunyn-Schmiedeberg's Arch Pharmacol 319:R69
Gross G, Brodde OE, Schümann HJ (1981) Regulation of α 1-adrenoceptors in the cerebral cortex of the rat by thyroid hormones. Naunyn-Schmiedeberg's Arch Pharmacol 316:45–50
Harrison TS (1964) Adrenal medullary and thyroid relationships. Physiol Rev 44:161–185
Hornung R, Presek P, Glossmann H (1979) Alpha adrenoceptors in rat brain: direct identification with prazosin. Naunyn-Schmiedeberg's Arch Pharmacol 308:223–230
Ishac EJN, Pennefather JN, Handberg GM (1983) Effect of changes in thyroid state on atrial α- and β-adrenoceptors, adenylate cyclase activity, and catecholamine levels in the rat. J Cardiovasc Pharmacol 5:396–405
Jondorf WR, Grünberger D (1967) Comparison of cycloheximide effect on hepatic protein synthesis at the microsomal level in vivo and in vitro. Biochem Pharmacol 16:2055–2059
Karliner JS, Barnes P, Hamilton CA, Dollery CT (1979) Alpha1-adrenergic receptors in guinea pig myocardium: identification by binding of a new radioligand, (3H)-prazosin. Biochem Biophys Res Comm 1:142–149
Koerner D, Surks MT, Oppenheimer JH (1974) In vitro demonstration of specific triiodothyronine binding sites in rat liver nuclei. J Clin Endocrin Metab 38:706–709
Kunos G (1977) Thyroid hormone-dependent interconversion of myocardial α- and β-adrenoceptors in the rat. Br J Pharmacol 59:177–189
Kunos G, Vermes-Kunos I, Nickerson M (1974) Effects of thyroid state on adrenoceptor properties. Nature 250:779–781
Kunos G, Mucci L, O'Regan S (1980) The influence of hormonal and neuronal factors on rat heart adrenoceptors. Br J Pharmacol 71:371–386
Kuschke HJ (1960) Sympatho-adrenal activity in thyrotoxicosis. Br Med J I:1651
Landsberg L, Axelrod J (1968) Influence of pituitary thyroid and adrenal hormones on norepinephrine turnover and metabolism in the rat heart. Circ Res 22:559–571
Lau CH, Slotkin TA (1979) Accelerated development of rat sympathetic neurotransmission caused by neonatal triiodothyronine administration. J Pharmacol Exp Ther 208:485–490
Lau CH, Slotkin TA (1982) Maturation of sympathetic neurotransmission in the rat heart. VIII. Slowed development of noradrenergic synapses resulting from hypothyroidism. J Pharmacol Exp Ther 220:629–636
Leak DS, Brunjes WJ, Johns J, Starr P (1962) Adrenal medullary response to insulin hypoglycemia in hypothyroid patients. J Lab Clin Med 60:811–817
Lemmer B, Saller R (1974) Influence of light and darkness on the turnover of noradrenaline in the rat heart. Naunyn-Schmiedeberg's Arch Pharmacol 282:75–84
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
MacLeod KM, McNeill JH (1981) The influence of altered thyroid hormone levels on guinea pig cardiac adrenoceptors and histamine receptors. Can J Physiol Pharmacol 59:1039–1049
McConnaughey MM, Jones LR, Watanabe AM, Besch HR, Williams LT, Lefkowitz RJ (1979) Thyroxine and propylthiouracil effects on alpha- and beta-adrenergic receptor number, ATPase activities, and sialic acid content of rat cardiac membrane vesicles. J Cardiovasc Pharmacol 1:609–623
Minneman KP, Hegstrand LR, Molinoff PB (1979) Simultaneous determination of beta-1- and beta-2-adrenergic receptors in tissues containing both receptor subtypes. Mol Pharmacol 16:34–36
Minneman KP, Pittman RN, Molinoff PB (1981) β-Adrenergic receptor subtypes: properties, distribution, and regulation. Ann Rev Neurosci 4:419–461
Nagel M, Schümann HJ (1980) A sensitive method for determination of conjugated catecholamines in blood plasma. J Clin Chem Clin Biochem 18:431–432
Nagel-Hiemke M, Gross G, Lues I, Schümann HJ (1981) Influence of hypo- and hyperthyroidism on plasma catecholamines in pithed rats. Naunyn-Schmiedeberg's Arch Pharmacol 317: 159–164
Nakashima M, Hagino Y (1972) Evidence for the existence of alpha adrenergic receptor in isolated rat atria. Jpn J Pharmacol 22:227–233
Nakashima M, Tsuru H, Shigei T (1973) Stimulant action of methoxamine in the isolated atria of normal and 6-propyl-2-thiouracil-fed rats. Jpn J Pharmacol 23:307–312
Oppenheimer JH, Schwartz HL, Surks MJ, Koerner D, Dillmann WH (1976) Nuclear receptors and the initiation of thyroid hormone action. Recent Progr Horm Res 32:529–565
Peuler JD, Johnson GH (1975) A sensitive radioenzymatic assay of plasma catecholamines: initial studies in supine normotensive subjects. Clin Res 23:474–478
Pittman CS, Pittman JA (1974) Relation of chemical structure to the action and metabolism of thyroactive substance. In: Greep RO, Astwood EB, Greer MH, Solomon DH, Geiger SR (eds) Handbook of Physiology. Endocrinology, vol 3, Am Physiol Soc. Washington, pp 233–253
Prange AJ Jr, Meek JL, Lipton MA (1970) Catecholamines: diminished rate of synthesis in rat brain and heart after thyroxine pretreatment. Life Sci 9:901–907
Preiksaitis HG, Kan WH, Kunos G (1982) Decreased α 1-adrenoceptor responsiveness and density in liver cells of thyroidectomized rats. J Biol Chem 257:4321–4327
Rub HP, Thommen H, Porzig H (1981) Quantitative changes in β-adrenergic responses of isolated atria from hyper- and hypothyroid rats. Experientia 37:399–401
Scatchard G (1949) The attractions of proteins for small molecules and ions. Ann NY Acad Sci 51:660–672
Schümann HJ (1980) Are there α-adrenoceptors in the mammalian heart? Trends Pharmacol Sci 1:195–197
Schümann HJ, Borowski E, Gross G (1979) Changes of adrenoceptor-mediated responses in the pithed rat during propylthiouracil induced hypothyroidism. Eur J Pharmacol 56:145–152
Sharma VK, Banerjee SP (1978) α-Adrenergic receptor in rat heart. J Biol Chem 253:5277–5279
Sharma RV, Habhab O, Bhalla RC (1979) Effect of propylthiouracil treatment on the regulation of adenylate cyclase activity in rat myocardium. Biochem Pharmacol 28:2858–2860
Smith PG, Mills E, Slotkin T (1981) Maturation of sympathetic neurotransmission in the efferent pathway to the rat heart: ultrastructural analysis of ganglionic synaptogenesis in euthyroid and hyperthyroid neonates. Neuroscience 6:911–918
Spector S, Sjoerdsma A, Udenfriend S (1965) Blockade of endogenous norepinephrine synthesis by alpha-methyl-tyrosine, an inhibitor of tyrosine hydroxylase. J Pharmacol Exp Ther 147:86–95
Stiles GL, Caron MC, Lefkowitz J (1984) β-Adrenergic receptors: biochemical mechanism of physiological regulation. Physiol Rev 64:661–743
Stoffer S, Jiang NS, Gorman C, Pikler G (1973) Plasma catecholamines in hypothyroidism and hyperthyroidism. J Clin Endocrinol Metab 36:587–589
Su YF, Cubeddu-Ximenez L, Perkins JP (1976) Regulation of adenosine-3′,5′-monophosphate content of human astrocytoma cells: desensitization to catecholamines and prostaglandins. J Cyclic Nucleotide Res 2:257–270
Tse J, Wrenn RW, Kuo JF (1980) Thyroxine-induced changes in characteristics and activities of β-adrenergic receptors and adenosine 3′,5′-monophosphate and guanosine 3′,5′-monophosphate systems in the heart may be related to reputed catecholamine supersensitivity in hyperthyroidism. Endocrinology 107:6–16
Turner CW, Shenfield GM (1980) The effect of thyroid dysfunction on the chronotropic response to noradrenaline. Eur J Pharmacol 68:295–303
Wagner J, Brodde OE (1978) On the presence and distribution of α-adrenoceptors in the heart of various mammalian species. Naunyn-Schmiedeberg's Arch Pharmacol 302:239–254
Waynforth HB (1980) Experimental and surgical technique in the rat. Academic Press, London
Williams RS, Lefkowitz RJ (1979) Thyroid hormone regulation of alpha-adrenergic receptors: studies in rat myocardium. J Cardiovasc Pharmacol 1:181–189
Williams LT, Lefkowitz RJ, Watanabe AM, Hathaway DR, Besch HR Jr (1977) Thyroid hormone regulation of β-adrenergic receptor number. J Biol Chem 252:2787–2789
Williams RS, Dukes DF, Lefkowitz RJ (1981) Subtype specificity of α-adrenergic receptors in rat heart. J Cardiovasc Pharmacol 3:522–531
Wiswell J, Hurwitz G, Coronho V, Bing O, Child D (1963) Urinary catecholamines and their metabolites in hyperthyroidism and hypothyroidism. J Clin Endocrinol Metab 23:1102–1106
Yamada S, Yamamura H, Roeske W (1980) Alterations in cardiac autonomic receptors following 6-hydroxydopamine treatment in rats. Mol Pharmacol 18:185–192
Yeh SDJ, Shils ME (1969) Quantitative aspects of cycloheximide inhibition of amino acid incorporation. Biochem Pharmacol 18:1919–1926
Author information
Authors and Affiliations
Additional information
This work was supported by the Deutsche Forschungsgemeinschaft
Rights and permissions
About this article
Cite this article
Gross, G., Lues, I. Thyroid-dependent alterations of myocardial adrenoceptors and adrenoceptor-mediated responses in the rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 329, 427–439 (1985). https://doi.org/10.1007/BF00496378
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00496378