Summary
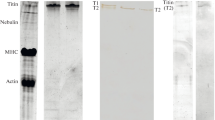
In previous work on rat striated muscle cells a sliver-reducing component was found selectively localized at the terminal cistern/transverse tubule system (Tandler and Pellegrino de Iraldi 1989). To further investigate that problem we performed the Hg−Ag argentaffin reaction on a sarcoplasmic reticulum fraction from rat skeletal muscle. Circular profiles corresponding to vesicular structures were found outlined by silver grains. The number of silver “stained” vesicles were less than the total number vesicles stained by conventional procedures. The correlation between argentaffinities in the intact muscle fiber and their subcellular organelles indicated that the Hg−Ag reactive vesicles must be those derived from the terminal cisternae of the sarcoplasmic reticulum. The silver-reducing constituent aggregates in the presence of 1 mM CaCl2 or 0.5 M K cacodylate. The state of aggregation induced by Ca2+ was not affected by incubation with 0.5% Triton X-100 or by 2 mM EDTA, thus suggesting a localization at or near the membrane of the terminal cistern vesicle facing the junctional gap. In Laemmli SDS-acrylamide gels the Hg−Ag reaction stained all proteins in a manner similar to Coomasie blue. It is suggested that the selective histochemical staining is the result of differential reactivities due to steric requirements of the chemical reaction.
Similar content being viewed by others
References
Antoniu B, Kim DH, Morii M, Ikemoto N (1985) Inhibitors of Ca2+ release from the isolated sarcoplasmic reticulum I. Ca2+ channel blockers. Biochim. Biophys Acta 816:9–17
Bean BP (1986) Calcium channels in skeletal muscle. What do they do? TINS November-December, pp 535–536
Brunschwig JP, Brandt, N, Caswell AH, Lukeman DS (1982) Ultrastructural observations of isolated intact and fragmented junctions of skeletal muscle by use of tannic acid mortanting. J Cell Biol 93:533–542
Cadwell JJS, Caswell AH (1982) Identification of a constituent of the junction feet linking terminal cisternae to transverse tubules in skeletal muscle. J Cell Biol 93:543–550
Campbell KP, Mac Lennan DH, Jorgensen AO (1983) Staining of the Ca2+-binding proteins, calsequestrin, calmodilin, troponin C, and S-100, with the cationic carbocyanine dye “stainsall”. J Biol Chem 258:11267–11273
Caswell AH, Brunschwig JP (1984) Identification and extraction of proteins that compose the triad junction of skeletal muscle. J Cell Biol 99:929–939
Caswell AH, Lau YH, Garcia M, Brunschwig JP (1979) Recognition and junction formation by isolated transverse tubules and terminal cisternae of skeletal muscle. J Biol Chem 254:202–208
Costello B, Chadwick C, Saito A, Chu A, Maurer A, Fleischer S (1986) Characterization of the junctional face membrane from terminal cisternae of sarcoplasmic reticulum. J Cell Biol 103:741–753
Fleischer S, Ogunbunni EM, Dixon MC, Fleer EAM (1985) Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci USA 82:7256–7259
Fleischer S, Saito A, Inui M (1987) Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem 262:15637–15642
Franzini-Armstrong C, Peachey LD (1981) Striated muscle. Contractile and control mechanisms. J Cell Biol 91:166–186
Franzini-Armstrong C, Kenney LJ, Varriano-Murston E (1987) The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J Cell Biol 105:49–56
Jorgensen AO, Kalnins V, Mac Lennan DH (1979) Localization of sarcoplasmic reticulum proteins in rat skeletal muscle by immunofluorescence. J Cell Biol 80:372–384
Jorgensen AO, Shen ACY, Campbell KP, Mac Lennan DH (1983) Ultrastructural localization of calsequestrin in rat skeletal muscle by immunoferritin labeling of ultrathin frozen sections. J Cell Biol 97:1573–1581
Kawamoto RM, Brunschwig JP, Kyungsook CK, Caswell AH (1986) Isolation, characterization, and localization, of the spanning protein from skeletal muscle triads. J. Cell Biol 103:1405–1414
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurements with the Folin phenol reagent. J Biol Chem 193:265–275
Meissner G (1975) Isolation and characterization of two types of sarcoplasmic reticulum. Biochim Biophys Acta 389:51–68
Saito A, Seiler S, Chu A, Fleischer S (1984) Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol 99:875–885
Somlyo AP (1985) The messenger across the gap. Nature 316:298–299
Somlyo Av, Gonzalez-Serratos H, Shuman H, McClellan G, Somlyo AP (1981) Calcium release and ionic exchanges in the sarcoplasmic reticulum of tetanized muscle: an electron probe study. J Cell Biol 90:577–594
Tandler CJ (1955) The reaction of nucleoli with ammoniacal silver nitrate in darkness; additional data. J Histochem Cytochem 3:196–202
Tandler CJ, Pellegrino de Iraldi A (1989) A silver-reducing component in rat striated muscle. I. Selective localization at the level of the terminal cistern/transverse tubule system. Histochemistry 92:15–22
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tandler, C.J., Gonzalez, D.A., Remorini, P.G. et al. A silver-reducing component in rat striated muscle. Histochemistry 92, 23–27 (1989). https://doi.org/10.1007/BF00495011
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00495011