Abstract
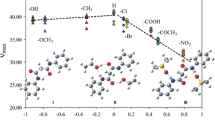
The IR and PMR spectra of an extensive series of methyl derivatives of aromatic and heteroaromatic compounds were investigated. With a few exceptions, the experimental data on the chemical shifts of the protons \((\delta ^{{\text{CH}}_{\text{3}} } )\) and the intensity of the band of the symmetrical stretching vibration [(ACH)1/2] for five- and six-membered heterorings can be united in a single reaction series with polysubstituted toluenes within the framework of an additive scheme. The (ACH)1/2 values correlate satisfactorily with the calculated (by the CNDO/2 method) total charges on the carbon and hydrogen atoms of the methyl group. In contrast to the intensities of the IR bands, linear relationships between the chemical shifts and the charges on the hydrogen atoms are observed only within the limits of particular reaction series. The lack of a unified relationship was interpreted as being the result of the effect of the ring current, the contribution of which to the \((\delta ^{{\text{CH}}_{\text{3}} } )\) value depends on the nature of the heteroatom.
Similar content being viewed by others
Literature cited
N. N. Zatsepina, I. F. Tupitsyn, and A. V. Kirova, Reakts. Sposobn. Org. Soedin., 9, 195 (1972).
I. F. Tupitsyn, N. N. Zatsepina, A. V. Kirova, and N. S. Kolodina, Reakts. Sposobn. Org. Soedin., 9, 207 (1972).
L. M. Jackman and S. Sternhell, Applications of NMR Spectroscopy in Organic Chemistry, Pergamon Press, New York (1969).
A. F. Cockerill, D. M. Rackham, and N. C. Franklin, J. Chem. Soc., Perkin II, No. 5, 509 (1973).
J. P. Morizer, Y. Pascal, and F. Vernier, Bull. Soc. Chim. France, No. 7, 2296 (1966).
S. Gronowitz and B. Gestblom. Arkiv Kemi. 18, 513 (1962).
J. Elguero, R. Jacquier, and H. C. N. Tien Duc, Bull. Soc. Chim. France, No. 12, 3727 (1966).
C. L. Habraken, H. J. Munter, and J. C. P. Westgeest, Rec. Trav. Chim., 86, 56 (1967).
D. N. Kravtsov, L. A. Fedorov, A. S. Peregudov, and A. N. Nesmeyanov, Dokl. Akad. Nauk SSSR, 196, 111 (1971).
J. H. Bowie, R. F. Donaghue, and H. J. Rodda, J. Chem. Soc., B, No. 9, 1122 (1969).
S. D. Sokolov, I. M. Yudintseva, and P. V. Petrovskii, Zh. Org. Khim., 6, 2584 (1970).
E. J. Vincent, R. Phan-Tan-Luu, J. Metzger, and J. M. Surzur, Bull. Soc. Chim. France, No. 11, 3524 (1966).
H. J. M. Dou, A. Friedmann, G. Vernin, and J. Metzger, Compt. Rend., C, 266, 714 (1968).
C. Richard and J. Anderson, J. Heterocycl. Chem., 1, 279 (1964).
H. Staab and A. Mannschreck, Chem. Ber., 98, 1111 (1965).
A. Perichand, J. C. Poite, and G. Mille, Bull. Soc. Chim. France, No. 10, 3830 (1972).
H. Markgraf and W. T. Bachmann, J. Org. Chem., 30, 3472 (1965).
L. A. Lee and J. W. Wheeler, J. Org. Chem., 37, 348 (1972).
R. E. Wasylishen, J. Rowbotham, J. Brian, and T. Schaefer, Can. J. Chem., 52, 833 (1974).
H. H. Jaffe and H. L. Jones, Advances in Heterocyclic Chemistry, Vol. 3 (1964), p. 209.
I. F. Tupitsyn, N. N. Zatsepina, N. S. Kolodina, and A. A. Kane, Reakts. Sposobn. Org. Soedin., 5, 931 (1968).
N. N. Zatsepina, I. F. Tupitsyn, Yu. L. Kaminskii, and N. S. Kolodina, Reakts. Sposobn. Org. Soedin., 6, 766 (1969).
G. Segal and M. Klein, J. Chem. Phys., 47, 4236 (1967).
R. Bruns, J. Chem. Phys., 58, 1849, 2585 (1973).
T. P. Lewis and I. W. Levin, Theor. Chim. Acta, 19, 55 (1970).
I. W. Levin, J. Chem. Phys., 55, 5393 (1971).
R. T. C. Brownlee, A. R. Katritzky, M. V. Sinnot, M. Szafran, R. D. Topsom, and L. Yakhontov, J. Am. Chem. Soc., 92, 6850 (1970).
R. T. C. Brownlee, J. A. Munday, R. D. Topsom, and A. R. Katritzky, J. Chem. Soc., Faraday II, No. 3, 349 (1973).
R. T. C. Brownlee, D. G. Cameron, R. D. Topsom, A. R. Katritzky, and A. J. Sparrow, J. Mol. Struct., 16, 365 (1973).
U. Pouchan, A. Dargelos, G, Ford, R. D. Topsom, and A. R. Katritzky, J. Chim. Phys., Phys.-Chim. Biol., 71, 934 (1974).
T. B. Grindley, K. F. Johnson, A. R. Katritzky, H. J. Keogh, C. Thirkettle, R. T. C. Brownlee, J. A. Munday, and R. D. Topsom, J. Chem. Soc., Perkin II, No. 3, 276 (1974).
B. Bäk, D. Christensen, Z. H. Hygeard, and J. R. Andersen, J. Mol. Spectr., 7, 58 (1961).
B. Bäk, D. Christensen, and W. Dixon, J. Mol. Spectr., 9, 124 (1962).
F. K. Larsen, M. S. Lehmann, J. Sotofte, and S. E. Rasmussen, Acta Chem. Scand., 24, 3248 (1970).
J. Berthon, J. Elguero, and C. Rerat, Acta Cryst., B, 26, 1820 (1970).
K. Bolton, R. D. Brown, F. R. Burden, and A. Mishra, J. Mol. Struct., 27, 261 (1975).
F. A. Momany and R. A. Bonham, J. Am. Chem. Soc., 86, 162 (1964).
Z. Nygaard, E. Asmussen, J. H. Hog, R. C. Maheshwari, C. H. Nielsen, U. B. Petersen, J. Rastrup-Andersen, and G. O. Sorensen, J. Mol. Struct., 8, 225 (1971).
J. Ambats and R. E. Marsh, Acta Cryst., 19, 942 (1965).
R. Michel, Frank D'Amato, and B. Marc, J. Mol. Struct., 9, 183 (1971).
J. A. Pople and M. Gordon, J. Am. Chem. Soc., 89, 4253 (1967).
J. Niwa, Bull. Chem. Soc. Jpn., 48, 118 (1975).
D. A. Dowson, G. K. Hamer, and W. F. Reynolds, Can. J. Chem., 52, 41 (1974).
G. Barbieri, R. Benassi, P. Lazzeretti, L. Schenetti, and F. Taddei, Org. Magn. Res., 7, 451 (1975).
J. A. Eldvice and L. M. Jackman, J. Chem. Soc., No. 7, 859 (1961).
F. Fringuelli, G. Marino, A. Tatiechi, and G. Grandolini, J. Chem. Soc., Perkin II, No. 4, 332 (1974).
S. Clementi, P. P. Forsythe, C. D. Johnson, A. R. Katritzky, and B. Terem, J. Chem. Soc., Perkin II, No. 4, 399 (1974).
Zh. F. Labarr and F. Galla, Usp. Khim., 40, 654 (1971).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1110–1119, August, 1977.
Rights and permissions
About this article
Cite this article
Zatsepina, N.N., Tupitsyn, I.F., Belyashova, A.I. et al. Study of the electron interactions of polysubstituted azoles by PMR and IR spectroscopy. Chem Heterocycl Compd 13, 894–903 (1977). https://doi.org/10.1007/BF00488919
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00488919