Abstract
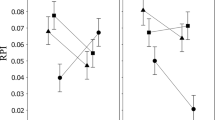
The activity levels of alcohol dehydrogenase and α-glycerophosphate dehydrogenase were compared among nine species of Drosophila representing three phylogenetic groups. For any given life stage, interspecific variability in activity level was much greater for ADH than for α-GPDH. Patterns of ontogenetic expression of enzyme activity were also much more variable among species for ADH than for α-GPDH. These results are consistent with the interpretation that α-GPDH is involved with a relatively uniform adaptive function among species, whereas ADH levels may reflect variable adaptive capabilities. There is a significant correlation between ADH activities and survivorship on alcohol-treated media for these nine species.
Similar content being viewed by others
References
Avise, J. C., and McDonald, J. F. (1975). Enzyme changes during development of holo-and hemi-metabolic insects. J. Comp. Biochem. & Physiol. (in press).
Birley, A. J., and Barnes, B. W. (1973). Genetical variation for enzyme activity in a population of Drosophila melanogaster. Heredity 31413.
Chefurka, W. (1965). Intermediary metabolism of carbohydrates in insects. In Rockstein, M. (ed.), Physiology of insects, Vol. 2, Academic Press, New York, p. 581.
David, J., Fouillet, P., and Arens, M. F. (1974). Comparaison de la sensibilité à l'alcool éthilique de six espèces de Drosophila du sous-groupe melanogaster. Arch. Zool. Exp. Gen. 115401.
Gibson, J. (1970). Enzyme flexibility in Drosophila melanogaster. Nature 227959.
Hewitt, N. E., Pipkin, S. B., Williams, N., and Chakrabarty, P. K. (1974). Variation in ADH activity in class I and class II strains of Drosophila. J. Hered. 65141.
Imberski, R. B., and Strommen, C. (1972). Developmental changes in alcohol dehydrogenase activity in Drosophila hydei. Drosophila Inform. Serv. 4874.
Johnson, G. B. (1974). Enzyme polymorphism and metabolism. Science 18428.
Karlson, P., and Sekeris, C. E. (1964). Biochemistry of insect metamorphosis. In Florkin, M., and Musen, H. S. (eds.), Comparative Biochemistry, Vol. VI, Academic Press, New York.
Kojima, K., Gillespie, J., and Tobari, Y. N. (1970). A profile of Drosophila species' enzymes assayed by electrophoresis. I. Number of alleles, heterozygosities, and linkage disequilibrium in glucose metabolizing systems and some other enzymes. Biochem. Genet. 4627.
McKenzie, J. A., and Parsons, P. A. (1972). Alcohol tolerance: An ecological parameter in the relative success of Drosophila melanogaster and Drosophila simulans. Oecologia 10373.
McKenzie, J. A., and Parsons, P. A. (1974). Microdifferentiation in a natural population of Drosophila melanogaster to alcohol in the environment. Genetics 77385.
O'Brien, S. J., and MacIntyre, R. J. (1972a). The α-glycerophosphate cycle in Drosophila melanogaster. I. Biochemical and developmental aspects. Biochem. Genet. 7141.
O'Brien, S. J., and MacIntyre, R. J. (1972b). The α-glycerophosphate in Drosophila melanogaster. II. Genetic aspects. Genetics 71127.
Patterson, J. T., and Stone, W. S. (1952). Evolution in the Genus Drosophila, Macmillan, New York.
Pipkin, S. B., and Hewitt, N. (1972). Variation of alcohol dehydrogenase levels in Drosophila species hybrids. J. Hered. 63267.
Pipkin, S. B., Rhodes, C., and Williams, N. (1973). Influence of temperature on Drosophila alcohol dehydrogenase polymorphism. J. Hered. 64181.
Rechsteiner, M. C. (1970). Drosophila lactate dehydrogenase and α-glycerophosphate dehydrogenase: Distribution and change in activity during development. J. Insect Physiol. 161179.
Sacktor, B. (1965). Energetics and respiratory metabolism of muscular contraction. In Rockstein, M. (ed.), Physiology of Insecta, Vol. 2, Academic Press, New York.
Sacktor, B., and Cockran, D. G. (1957). DPN-specific-α-glycerophosphate dehydroglucose in insect flight muscle. Biochim. Biophys. Acta 25649.
Ursprung, H., Sofer, W. H., and Burroughs, N. (1970). Ontogeny and tissue distribution of alcohol dehydrogenase in Drosophila melanogaster. Wilhelm Roux Arch. 164201.
Vigue, C. L., and Johnson, F. M. (1973). Isozyme variability in species of the genus Drosophila. VI. Frequency-property-environment relationships of allelic alcohol dehydrogenases in D. melanogaster. Biochem. Genet. 9213.
Ward, R. D. (1974). Alcohol dehydrogenase in Drosophila melanogaster: Activity variation in natural populations. Biochem. Genet. 12449.
Ward, R. D., and Herbert, P. D. N. (1972). Variability of alcohol dehydrogenase activity in a natural population of Drosophila melanogaster. Nature New Biol. 246243.
Author information
Authors and Affiliations
Additional information
This research was supported by Contract AT(04-3)-34 200 with ERDA. The authors are supported by an NIH training grant in genetics.
Rights and permissions
About this article
Cite this article
McDonald, J.F., Avise, J.C. Evidence for the adaptive significance of enzyme activity levels: Interspecific variation in α-GPDH and ADH in Drosophila . Biochem Genet 14, 347–355 (1976). https://doi.org/10.1007/BF00484773
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00484773