Abstract
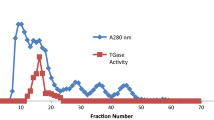
A method for the starch gel electrophoresis of human L-glutamate dehydrogenase (GLUD) is described, as is the tissue distribution of GLUD detected by this method. Extracts of livers from 200 Whites were analyzed without demonstration of an electrophoretic variant. The molecular size was estimated to be 330,000 and the isoelectric point pH 4.83.
Similar content being viewed by others
References
Bayley, P. M., and Radda, G. K. (1966). Conformational changes and the regulation of glutamate dehydrogenase activity. Biochem. J. 98105.
Blumenthal, K. M., and Smith, E. L. (1975). Alternative substrates for glutamate dehydrogenase. Biochem. Biophys. Res. Commun. 6278.
Chabner, B. A., Johns, D. G., Coleman, C. N., Drake, J. C., and Evans, W. H. (1974). Purification and properties of cytidine deaminase from normal and leukemic granulocytes. J. Clin. Invest. 53922.
Colman, R. F., and Frieden, C. (1966). On the role of amino groups in the structure and function of glutamate dehydrogenase. J. Biol. Chem. 2413661.
Engel, P. C. (1973). Evolution of enzyme regulator sites: Evidence for partial gene duplication from amino-acid sequences of bovine glutamate dehydrogenase. Nature 241118.
Grimes, H., and Fottrell, P. F. (1966). Enzymes involved in glutamate metabolism in legume root nodules. Nature 212295.
Malcolm, A. D. B., and Radda, G. K. (1968). Allosteric transitions of glutamate dehydrogenase. Nature 219947.
Markert, C. L., and Moeller, F. (1959). Multiple forms of enzymes: tissue ontogenic, and species specific patterns. Proc. Natl. Acad. Sci. 45753.
McGivan, J. D., Bradford, N. M., Crompton, M., and Chappell, J. B. (1973). Effect of L-leucine on the nitrogen metabolism of isolated rat liver mitochondria. Biochem. J. 134209.
Puranen, J., and Arstila, A. (1967). Inhibition of glutamic dehydrogenase by clomiphene. Nature 21378.
Teng, Y. S., Anderson, J. E., and Giblett, E. R. (1975). Cytidine deaminase: A new genetic polymorphism demonstrated in human granulocytes. Am. J. Hum. Genet. 27492.
Thurman, D. A., Palin, C., and Laycock, M. V. (1965). Isoenzymatic nature of L-glutamic dehydrogenase of higher plants. Nature 207193.
Tudball, N., Bailey-Wood, R., and Thomas, P. (1972). The role of histidine residues in glutamate dehydrogenase. Biochem. J. 129419.
van der Helm, H. J. (1962). L-Glutamate dehydrogenase isoenzymes. Nature 194773.
Wallis, R. B., and Holbrook, J. J. (1973). The reaction of histidine residue in glutamate dehydrogenase with diethyl pyrocarbonate. Biochem. J. 133183.
Wooton, J. C. (1974). The coenzyme-binding domains of glutamate dehydrogenases. Nature 252542.
Author information
Authors and Affiliations
Additional information
This investigation was supported by Public Health Service Grant No. 1 F22 CAO2083-01 of the National Cancer Institute.
Rights and permissions
About this article
Cite this article
Nelson, R.L., Povey, M.S., Hopkinson, D.A. et al. Electrophoresis of human L-glutamate dehydrogenase: Tissue distribution and preliminary population survey. Biochem Genet 15, 87–91 (1977). https://doi.org/10.1007/BF00484550
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00484550