Abstract
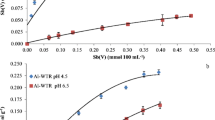
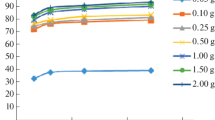
The retention of antimony (a potential toxin) in polluted soils or waterway sediments can involve interaction with several component phases. One of these, humic acid, has been found to adsorb antimony (III) from solutions of Sb(OH)3 or potassium antimonyl tartrate (C8H4K2Sb2O12) in accordance with Langmuir type isotherms. Using Sb(OH)3 solutions (initial Sb levels < 10 μM) the bonding constant value (at pH 4) was 6 × 105, with a calculated saturation capacity of 23 μmol g−1. In the antimonyl tartrate systems (initial Sb levels 0.5 to 75 μM) the bonding constant value for the sorbed species was 1.6 × 105 and the saturation capacity 53 μmol g−1. Addition of small amounts of HCl or NaOH (to vary the pH between 3.1 and 5.4) had little effect on the amount sorbed from KSbT solutions but with Sb(OH)3 solutions uptake was reduced (by about 15%). In the presence of NaCl (0.5 or 0.05M) Sb uptake increased (by about 15%). Antimony (V) (introduced as KSb (OH)6) was not sorbed from solutions < 10 μM in this salt. Using more concentrated solutions, uptake gradually increased, reaching a plateau value of around 8 μmol g−1 with solutions initially 50 or 75 μM.
Similar content being viewed by others
References
Boyle, R. W. and Jonasson, I. R.: 1973, J. Geochem. Explor. 2, 251.
Brannon, J. M. and Patrick, W. H.: 1985, Environ. Pollut. Ser. B. 9, 107.
Crecelius, E. A., Bothner, M. H. and Carpenter, R.: 1975, Environ. Sci. Techol. 9, 325.
Gate, S. H. and Richardson, E.: 1961, J. Inorg. Nucl. Chem. 23, 97.
Gate, S. H. and Richardson, E.: 1961, J. Inorg. Nucl. Chem. 23, 265.
Gayer, K. H. and Garrett, A. B.: 1952, J. Amer. Chem. Soc. 74, 2352.
Ghoda, S.: 1975, Bull. Chem. Soc. Jpn. 48, 1213.
Gilbert, T. R. and Hume, D. N.: 1973, Anal. Chim. Acta. 65, 451.
Ragaini, R. C., Raison, H. R. and Roberts, N.: 1977. Environ. Sci. Technol. 11, 773.
Strohal, P., Huljev, D., Lulic, S. and Picer, M.: 1975, Estuarine Coastal Mar. Sci. 3, 119.
Thanabalasingam, P. and Pickering, W. F.: 1985. Environ. Pollut. Ser. B. 9, 267.
Thanabalasingam, P. and Pickering, W. F.: 1986. Environ. Pollut. Ser. B. 12, 233.
Thanabalasingam, P. and Pickering, W. F.: 1990, Water. Air, and Soil Pollut. 49, 175.
Waller, P. A. and Pickering, W. F.: 1991, Chem. Spec. Bioavail. 3, 9.
Waller, P. A. and Pickering, W. F.: 1995, Talanta 5, 42, 197.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pilarski, J., Waller, P. & Pickering, W. Sorption of antimony species by humic acid. Water Air Soil Pollut 84, 51–59 (1995). https://doi.org/10.1007/BF00479588
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00479588