Abstract
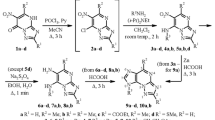
5-Amino-6-chloro-4-dihydroxyalkylaminopyrimidines, which are cyclized to 6-chloro-9-(dihydroxyalkyl)purines, were obtained by the condensation of 5-amino-4,6-dichloropyrimidine with 2-amino-1,3-dihydroxypropane or with 2-amino-1,4-dihydroxybutane. The corresponding 6-hydroxy and 6-amino derivatives were obtained by replacement of the chlorine.
Similar content being viewed by others
Literature cited
S. A. Giller, in: Modern State of the Chemotherapy of Malignant Tumors [in Russian], Riga (1968), p. 19.
I. A. Montgomery and C. Temple, J. Am. Chem. Soc., 79, 5239 (1957).
H. T. Schaeffer, D. Vogel, and R. Vince, J. Med. Chem., 8, 502 (1965).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 2, pp. 262–264, February, 1971.
Rights and permissions
About this article
Cite this article
Lidak, M.Y., Shluke, Y.Y., Zarinya, B.V. et al. Synthesis of 6-substituted 9-(dihydroxyalkyl) purines. Chem Heterocycl Compd 7, 240–242 (1971). https://doi.org/10.1007/BF00473098
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00473098