Abstract
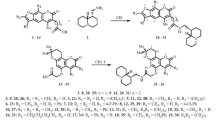
A new variant of the synthesis of 7-amino-4-methyl-coumarin from m-aminophenol via a three-step scheme is proposed. Acylation of m-aminophenyl with methoxycarbonyl chloride gave m-(N-methoxycarbonylamino)phenol, which was converted to 7-(N-methoxycarbonyl-amino)-4-methylcoumarin by condensation with acetoacetic ester in sulfuric acid. Heating of the coumarin with concentrated alkali leads to an intermediate, which, after acidification, is converted to 7-amino-4-methylcoumarin in high yield.
Similar content being viewed by others
Literature Cited
M. Zimmerman, E. Yurewicz, and G. Patel. Anal. Biochem., 70, 258 (1976).
A. Baici, P. Salgam, K. Fehr, and A. Böni, Biochem. Pharm., 30, 703 (1981).
I. I. Grandberg, L. K. Denisov, and O. A. Popova, Khim. Geterotsikl. Soedin., No. 2, 147 (1987).
H. Pechmann and O. Schwarz, Berichte, 32, 3696 (1899).
E. Bissel, A. Mitchell, and R. Smith, J. Org. Chem., 45, 2283 (1980).
M. V. Rubtsov and A. G. Baichikov, Synthetic Pharmaceutical-Chemical Preparations [in Russian], Meditsina, Moscow (1971).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 3, pp. 312–314, March, 1990.
Rights and permissions
About this article
Cite this article
Pozdnev, V.F. Improved method for synthesis of 7-amino-4-methylcoumarin. Chem Heterocycl Compd 26, 264–265 (1990). https://doi.org/10.1007/BF00472539
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00472539