Abstract
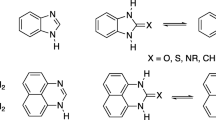
The UV, PMR, and ESR spectra as well as the magnetic susceptibilities of 1- and 2- substituted perimidine, aceperimidine, and a number of compounds related to them were measured and discussed. The results obtained indicate that the distribution of the π-electron density in the perimidine system is very nonuniform, which is in agreement with quantum-mechanical calculations. This is particularly manifested in the depressed diamagnetism of perimidines, the presence of an ESR signal for them, and in the shift of the chemical shifts of the protons to high field. Owing to the high-lying upper occupied molecular orbital, the perimidines are extremely strong π donors and readily form charge-transfer complexes.
Similar content being viewed by others
Literature cited
I. S. Kashparov and A. F. Pozharskii, Khim. Geterotsikl. Soedin., 124 (1971).
V. I. Minkin, Yu. A. Zhdanov, I. D. Sadekov, O. A. Raevskii, and A. D. Garnovskii, Khim. Geterotsikl. Soedin., 1100 (1967).
A. F. Pozharskii and E. N. Malysheva, Khim. Geterotsikl. Soedin., 103 (1970).
A. F. Pozharskii and I. S. Kashparov, Khim. Geterotsikl. Soedin., 111 (1970).
A. F. Pozharskii and I. S. Kashparov, Khim. Geterotsikl. Soedin., 1129 (1970).
N. P. Buu-Hoi, P. Jacquinon, and M. Marty, Bull. Soc. Chim. France, 461 (1960).
K. J. Morgan, J. Chem. Soc., 2343 (1961).
L. L. Popova, I. D. Sadekova, and V. I. Minkin, Reakts. Sposobnost' Organ. Soedin., Tartu, 6, 47 (1969).
S. Castellano and A. A. Bothner-By, J. Chem. Phys., 41, 3863 (1964).
L. A. Blyumenfel'd, V. V. Voevodskii, and A. G. Semenov, Use of Electron Paramagnetic Resonance in Chemistry [in Russian], Izd. Sibirsk. Otd. AN SSSR, Novosibirsk (1962), pp. 89, 222.
J. H. Sharp, J. Phys. Chem., 70, 584 (1966).
V. Paragamian, M. B. Baker, B. M. Puma, and J. Reale, J. Heter. Chem., 5, 591 (1968).
V. I. Minkin and B. Ya. Simkin, Khim. Geterotsikl. Soedin. (1971) (in press).
V. Boekelheide and G. K. Vick, J. Am. Chem. Soc., 78, 653 (1956).
R. T. C. Brownlee, R. E. J. Hutchinson, A. R. Katritzky, T. T. Tidwell, and R. D. Topsom, J. Am. Chem. Soc., 90, 1757 (1968).
P. A. S. Smith, J. H. Hall, and R. O. Kan, J. Am. Chem. Soc., 84, 485 (1962).
B. Pullman and A. Pullman, Quantum Biochemistry, Wiley (1963).
R. Foster, Nature, 183, 1253 (1959).
R. Foster, Nature, 181, 337 (1958).
S. Streitwieser, in: Progress in Physical Organic Chemistry [Russian translation], Moscow (1967), p. 9.
A. F. Pozharskii, in: Outline of Azole Chemistry [in Russian], Izd. Rostovskogo Univ. (1965), p. 37.
Ya. G. Dorfman, Magnetism and Chemical Bonding [in Russian], Fizmatgiz (1961).
H. Prinzbach, V. Frendenberger, and U. Scheidegger, Helv. Chim. Acta, 50, 1087 (1967).
P. Tavs, H. Sieper, and H. Beecken, Ann., 704, 150 (1967).
B. M. Lunch and H. J. M. Don, Tetrah. Lett., 2627 (1966).
A. J. Jones, Rev. Pure and Appl. Chem., 18, 253 (1968).
Author information
Authors and Affiliations
Additional information
See [1] for communication V.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 4, pp. 543–552, April, 1971.
Rights and permissions
About this article
Cite this article
Pozharskii, A.F., Kashparov, I.S., Holls, P.J. et al. Heterocyclic pleiadiene analogs. Chem Heterocycl Compd 7, 507–515 (1971). https://doi.org/10.1007/BF00471496
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00471496