Summary
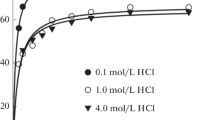
The reagent Bismuthiol II completely precipitates palladium at a maximum acidity of 0.3 N in nitric acid, 0.5 N in hydrochloric acid or 1 N in sulphuric acid and also at a maximum pH of about 8.0. The palladium complex Pd (C8H5N2S3)2 is stable even up to a temperature of about 250° C. From a mineral acid solution palladium can be estimated in presence of ions of Fe2+, Al, Cr, Th, Ce3+, Zr, Ti4+, UO2 2+, Be, Mn, Co, Ni, Mg, P04 3−, AsO4 3−, rare earths, alkalis, alkaline earths, Ce4+, WO4 2− and MoO4 2− that are not ordinarily precipitated by the reagent. At a pH of 4.75 to 8.2, EDTA, Na-salt, keeps in solution, besides the above ions, the ions of Tl+, Cu2+, Pb, Bi3+, As3+, Sb3+, Zn, Cd, Fe3+, CrO4 2−, AsO3 3− and VO3 −. Tartrate, however, at a pH 6.2–8, keeps all the ions including Sn4+ in solution except of course Tl+, Cu2+, Pb and Cd. Separation from Ce4+, WO4 2−, MoO4 2− and AsO4 3− at a low pH require the presence of tartrate. Ag+, Hg2+ and also Pb may be complexed with potassium iodide at a pH 6–8. Tl+ and Ag+ may also be separated in presence of cyanide in an acetate buffer when palladium remains in solution and from which it may be re-precipitated by acidification.
Similar content being viewed by others
References
Feigl, F.: Chemistry of Specific, Selective and Sensitive Reactions, Academic Press, Inc., New York, 1949, p. 8.
Flagg, J. F.: Organic Reagents, Interscience Publishers, Inc., New York, 1948, p. 155.
Majumdar, A. K.: J. Indian chem. Soc. 19, 396 (1942); 21, 347 (1944).
Majumdar, A. K., and M. M. Chakrabartty: Z. analyt. Chem. 154, 262 (1957).
Author information
Authors and Affiliations
Additional information
Part II see Z. analyt. Chem. 154, 413 (1957).
Rights and permissions
About this article
Cite this article
Majumdar, A.K., Chakrabartty, M.M. Bismuthiol II as an analytical reagent. Z. Anal. Chem. 155, 1–6 (1957). https://doi.org/10.1007/BF00461765
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00461765