Abstract
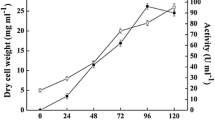
Succinyl-CoA synthetase from Saccharomyces cerevisiae was partially purified (20-fold) with a yield of 44%. The Michaelis-Menten constants were determined: K m (succinate)=17mm; K m (ATP)=0.13mm; K m (CoA)=0.03mm. The succinyl-CoA synthetase has a molecular weight of about 80000 dalton (as determined by polyacrylamide gradient gel electrophoresis). The pH optimum is at 6.0. During fermentation the activity of succinyl-CoA synthetase is lower than in aerobically grown yeast cells. The presence of succinyl-CoA synthetase in fermenting yeasts may be regarded as an indication for the oxidative formation of succinate. In fermenting yeast cells succinyl-CoA synthetase is repressed by glucose if ammonium sulphate serves as nitrogen source. This catabolite repression is not observed with disaccharides or when amino acids are used as nitrogen source.
Similar content being viewed by others
References
Bridger, W. A. 1974. Succinyl-CoA synthetase. p. 581–606. In P. D. Boyer (ed.), The enzymes, Vol. 10. — Academic Press, New York.
Cha, S. 1969. Succinate thiokinase from pig heart. p. 62–69. In S. P. Colowick and N. O. Kaplan (eds), Methods in enzymology, Vol. 13. — Academic Press, London, New York.
Cha, S. and Parks, R. E. 1964. Succinic thiokinase. I. Purification of the enzyme from pig heart. — J. Biol. Chem. 239: 1961–1967.
Chapman, C. and Bartley, W. 1968. The kinetics of enzyme changes in yeast under conditions that cause the loss mitochondria. — Biochem. J. 107: 455–465.
Gergely, J., Hele, P. and Ramakrishnan, C. V. 1952. Succinyl and acetyl coenzyme A deacylases. — J. Biol. Chem. 198: 323–334.
Hauber, J. and Singer, T. P. 1967. Studies on succinate dehydrogenase. 14. Intracellular distribution, catalytic properties and regulation of fumarate reductases in yeast. — Eur. J. Biochem. 3: 107–116.
Heerde, E. and Radler, F. 1978. Metabolism of the anaerobic formation of succinic acid by Saccharomyces cerevisiae. — Arch. Microbiol. 117: 269–276.
Holzer, H. 1977. Function and regulation of yeast intracellular proteinases. p. 21–22. In J. Oksanen and H. Suomalainen (eds), Proc. EUCHEM Conference on ‘Metabolic reactions in the yeast cell in anaerobic and aerobic conditions’, held in Matinkylä/Helsinki, Finland.
Kaufman, S. and Alivisatos, S. G. A. 1955. Purification and properties of the phosphorylating enzyme from spinach. — J. Biol. Chem. 216: 141–152.
Labbe, P. 1971. Synthèse du protohème par la levure Saccharomyces cerevisiae. I. Mise en évidence de différentes étapes de la synthèse du protohème chez la levure cultivée en aérobiose et en anaérobiose. Influence des conditions de culture sur cette synthèse. — Biochime 53: 1001–1014.
Moffet, F. J. and Bridger, W. A. 1970. The kinetics of succinyl coenzyme A synthetase from Escherichia coli. — J. Biol. Chem. 245: 2758–2762.
Nishimura, J. S., Mitchell, T. and Grinnell, F. 1973. Immunochemical studies of Escherichia coli succinic thiokinase; effects of sulfhydryl reagents and other treatments on the enzyme. —J. Biol. Chem. 248: 743–748.
Oura, E. 1977. Some characteristic features of the anaerobic metabolism of yeast. p. 23–25. In J. Oksanen and H. Suomalainen (eds), Proc. EUCHEM Conference on ‘Metabolic reactions in the yeast cell in anaerobic and aerobic condition’, held in Matinkylä/Helsinki, Finland.
Palmer, J. M. and Wedding, R. T. 1966. Purification and properties of succinyl-CoA synthetase from Jerusalem artichoke mitochondria. — Biochim. Biophys. Acta 113: 167–174.
Polakis, E. S. and Bartley, W. 1965. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. — Biochem. J. 97: 284–297.
Tsuboi, K. K. and Hudson, P. B. 1957. Enzymes of the human erythrocyte. I. Purine nucleoside phosphorylase, isolation procedure. — J. Biol. Chem. 224: 879–887.
Warburg, O. und Christian, W. 1941. Isolierung und Kristallisation des Gärferments Enolase. — Biochem. Z. 310: 384–421.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schwartz, H., Steitz, H.O. & Radler, F. Partial purification and characterization of succinyl-CoA synthetase from Saccharomyces cerevisiae . Antonie van Leeuwenhoek 49, 69–78 (1983). https://doi.org/10.1007/BF00457881
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00457881