Abstract
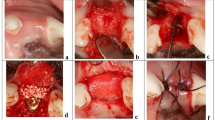
Forty-three patients at two different international sites underwent onlay facial augmentation of the malar, paranasal, and chin regions using 61 HTR polymer preformed implants. All implants were placed intraorally and rigidly fixed with a titanium screw. Over postoperative periods ranging from two to five years, one implant was removed because of infection. Two other implants in patients with rheumatic and connective tissue disease were removed because of persistent pain and erythema. Another peri-implant infection was treated successfully without removal. Oneyear postoperative radiographs in patients with chin implants demonstrated no underlying bone resorption. This porous polymeric material appears to offer clinical results comparable to other alloplastic materials for onlay facial skeletal augmentation.
Similar content being viewed by others
References
Ashman A, Bruins PF: A new immediate hard tissue replacement (HTR) for bone in the oral cavity. J Oral Implantol 10:419, 1982
Cook AD, Sagers RD, Pitt WG: Bacterial adhesion to poly(HEMA)-based hydrogels. J Biomed Mat Res 27:119, 1993
Donohue WB, Mascres C: A comparison of the effects of two hydroxyapatites and a methacrylate resin on bone formation in the rat ilium. Int J Oral Maxillofac Implants 8:75, 1993
Eppley BL, Sadove AM, German RZ: Evaluation of HTR polymer as a craniomaxillofacial graft material. Plast Reconstr Surg 86:1085, 1990
Fleer A, Verhoef J: An evaluation of the role of surface hydrophobicity and extracellular slime in the pathogenesis of foreign body-related infections due to coagulasenegative Staphylococci. J Invest Surg 2:391, 1989
Golomb G, Barashi A, Wagner D, Nachmias O: In-vitro and in-vivo models for the study of the relationship between hydrophilicity and calcification of polymeric and collagenous biomaterials. Clin Mat 13:61, 1993
Harkes C, Feijen J, Dankert J: Adhesion of Escherichia coli on to a series of poly(methacrylates) differing in charge and hydrophobicity. Biomaterials 12:853, 1991
Hinderer UT: Nasal base, maxillary, and infraorbital implants-alloplastic. Clin Plast Surg 18:87, 1991
Holmstrom H, Kahnberg K-E, Lessard L: The use of preformed HTR polymer implants for chin augmentation. A preliminary report. Scand J Plast Reconstr Hand Surg 27:1, 1993
Isaksson S, Alberius P, Klinge B: Influence of three alloplastic materials on calvarial bone healing. An experimental evaluation of HTR polymer, lactomer beads, and a carrier gel. Int J Oral Maxillofac Surg 22:375, 1993
Ludwicka A, Jansen B, Uhlenbruck G, Jeljaszewicz J, Pulverer G: Attachment of Staphylococci to modified and unmodified synthetic polymers. In: Ducheyne P, Van der Perre G, Aubert AE (eds): Biomaterials and Biomechanics. Amsterdam: Elsevier Scinece Publishers. 1984, pp 265–270
Mladick RA: Alloplastic cheek augmentation. Clin Plast Surg 18:29, 1991
Oga M, Sugioka Y, Hobgood CD, Gristina AG, Myrvik QN: Surgical biomaterials and differential colonization by Staphylococcus epidermidis. Biomaterials 9:285, 1988
Petty W, Spanier S, Shuster JJ, Silverthorne C: The influence of skeletal implants on incidence of infection. J Bone Joint Surg (Am) 67:1238, 1985
Ripamonti U, Petit J-C, Moehl T, van den Heever B, van Wyk J: Immediate reconstruction of massive cranioorbito-facial defects with allogeneic and alloplastic ma trices in baboons. J Craniomaxillofac Surg 21:302, 1993
Smetana K Jr, Vacik J, Souckova D, Pitrova S: The influence of chemical functional groups on implant biocompatibility. Clin Mat 13:47, 1993
Stahl SS, Fruom SJ, Tarnow D: Human clinical and histologic responses to the placement of HTR polymer particles in 11 intrabony lesions. J Periodontol 60:269, 1990
Uyen HMW, van der Mei HC, Weerkamp AH, Busscher HJ: Zeta potential and the adhesion of oral Streptococci to polymethylmethacrylate. Biomat Art Cells Art Org 17:385,1989
Wellisz T: Clinical experience with the Medpor porous polyethylene implant. Aesth Plast Surg 17:339, 1993
Whitaker LA: Aesthetic augmentation of the malarmidface structures. Plast Reconstr Surg 80:337, 1987
Whitaker LA: Aesthetic contouring of the facial support system. Clin Plast Surg 16:815, 1989
Whitaker LA, Pertschuk M: Facial skeletal contouring for aesthetic purposes. Plast Reconstr Surg 59:245, 1982
Wilkinson T: Complications in aesthetic malar augmentation. Plast Reconstr Surg 71:643, 1983
Yukna RA: HTR polymer grafts in human periodontal osseous defects. 1. 6-month clinical results. J Periodontol 61:633, 1990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eppley, B.L., Sadove, A.M., Holmstrom, H. et al. HTR® polymer facial implants: A five-year clinical experience. Aesth. Plast. Surg. 19, 445–450 (1995). https://doi.org/10.1007/BF00453878
Issue Date:
DOI: https://doi.org/10.1007/BF00453878