Abstract
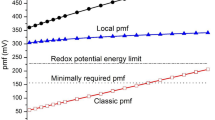
Whole cells of the extreme thermophile Thermus thermophilus HB8 contained a membrane-bound respiratory chain (comprised of nicotinamide nucleotide transhydrogenase, NADH dehydrogenase, menaquinone, and cytochromes b, c, aa3, o), which exhibited a maximum→H+/O quotient of approximately 8 g-ion H+·g-atom O-1 for the oxidation of endogenous substrates. Whole cell respiration at 70° at the expense of endogenous substrates or ascorbate-TMPD generated a transmembrane protonmotive force (Δp) of up to 197 mV and an intracellular phosphorylation poteintial (ΔGp), measured under similar conditions, of approximately 43.9 kJ·mol-1.
The measured ΔGp/Δp ratio thus indicated an→H+/ATP quotient of approximately 2.3 g-ion H+·mole ATP-1. Glucose-limited continuous cultures of T. thermophilus at 60°, 70° and 78.5° exhibited extremely low moler growth yields (Y maxO2 ≤27.6 g cells·mol O -12 ; Y maxglucose ≤64.4 g cells ·mol glucose-1) compared with mesophilic bacteria of similar respiratory chain composition and proton translocation efficiency. These low yields are probably at least partly explained by the extremely high permeability of the cytoplasmic membrane to H+, which thus causes the cells to respire rapidly in order to maintain the protonmotive force at a level commensurate with cell growth.
Similar content being viewed by others
Abbreviations
- TPMP+ :
-
triphenylmethylphosphonium cation
- FCCP:
-
carbonylcyanide p-trifluoromethoxy phenythydrazone
- TMPD:
-
N,N,N′,N′-tetramethyl-p-phenylene diamine
References
Ackrell BAC, Jones CW (1971) The respiratory system of Azotobacter vinelandii; 2, oxygen effects. Europ J Biochem 20:29–35
Baker K (1968) Low cost continuous culture apparatus. Lab Pract 17:817–824
Bakker EP (1978) Accumulation of thallous ions (T1+) as a measure of the electrical potential difference across the cytoplasmic membrane of bacteria. Biochemistry 17:2899–2904
Bishop DHL, Pandya KP, King HK (1962) Ubiquinone and vitamin K in bacteria. Biochem J 83:606–614
Booth IR, Mitchell WJ, Hamilton WA (1979) Quantitative analysis of proton-linked transport systems. Biochem J 182:687–696
Chicken E, Spode JA, Jones CW (1981) Respiration-linked proton translocation in the moderate thermophile Bacillus stearothermophilus. FEMS Microbiol Lett 11:181–185
Collins MD, Shah HN, Minnikin DE (1980) A note on the separation of natural mixtures of bacterial menaquinones using reverse phase thinlayer chromatography. J Appl Bacteriol 48:277–282
Colowick SP, Womack FC (1969) Binding of diffusible molecules by macromolecules; rapid measurement by rate of dialysis. J Biol Chem 244:774–777
Dawson MJ, Jones CW (1981) Respiration-linked proton translocation in the obligate methylotroph Methylophilus methylotrophus. Biochem J 194:915–924
Dawson AG, Chappell JB (1978) The oxidative activities of membrane vesicles from Bacillus caldolyticus. Biochem J 170:395–405
Degryse E, Glansdorff N, Pierard A (1978) A comparative analysis of extreme thermophilic bacteria belonging to the genus Thermus. Arch Microbiol 117:189–196
Downs AJ, Jones CW (1975) Energy conservation in Bacillus megaterium. Arch Microbiol 105:159–167
Erecinska M, Davis JS, Wilson DF (1979) Regulation of respiration in Paracoccus denitrificans; the dependence on redox state of cytochrome c and [ATP]/[ADP][Pi]. Arch Biochem Biophys 197:463–469
Farmer IS, Jones CW (1976) The energetics of Escherichia coli during aerobic growth in continuous cultures. Europ J Biochem 67:115–122
Fee JA, Findling KL, Lees A, Yoshida T (1978) Respiratory proteins of some extremely thermophilic bacteria. In: PL Dutton, TS Leigh and A Scarpa (eds) Frontiers of biological energetics vol. 1. Academic Press, London New York, pp 118–126.
Friedberg I, Kaback HR (1980) Electrochemical proton gradient in Micrococcus lysodeikticus cells and membrane vesicles. J Bacteriol 142:651–658
Hickey CW, Daniel RM (1979) The electron transport system of an extremely thermophilic bacterium. J Gen Microbiol 114:195–200
Hon-nami K (1979) A double-α c-type cytochrome, cytochrome c-555, 549 from an extreme thermophile, Thermus thermophilus HB8. J Biochem (Tokyo) 86:1687–1695
Hon-nami K, Oshima T (1980) Cytochrome oxidase from an extreme thermophile, Thermus thermophilus HB8. Biochem Biophys Res Commun 92:1023–1029
Itaya K, Ui M (1966) A new micromethod for the colorimetric determination of inorganic phosphate. Clinica Chimica Acta 14:361–366
Jones CW (1977) Aerobic respiratory systems in bacteria. In: BA Haddock, WA Hamilton (eds) Microbial energetics. Cambridge University Press, Cambridge. Soc Gen Microbiol Symp 27:23–59
Jones CW, Ackrell BAC, Erickson SK (1971) Respiratory control in Azotobacter vinelandii membranes. Biochim Biophys Acta 245:54–62
Jones CW, Brice JM, Edwards C (1977) The effect of respiratory chain composition on the growth efficiencies of aerobic bacteria. Arch Microbiol 115:85–93
Jones CW, Brice JM, Downs AJ, Drozd JW (1975) Bacterial respirationlinked proton translocation and its relationship to respiratory chain composition. Europ J Biochem 52:265–271
Kagawa Y (1978) Reconstitution of the energy transformer, gate and channel subunit reassembly, crystalline ATPase and ATP synthesis. Biochim Biophys Acta 505:45–93
Kashket ER, Barker SL (1977) Effects of potassium ions on the electrical and pH gradients across the membranes of Streptococcus lactis cells. J Bacteriol 130:1017–1023
Kenkel T, Trela JM (1979) Protein turnover in the extreme thermophile Thermus aquaticus. J Bacteriol 140:543–546
Kimmich GA, Randles J, Brand JS (1975) Assay of picomole amounts of ATP, ADP and AMP using the luciferase enzyme system. Anal Biochem 69:187–206
Kovacs N (1956) Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature (London) 178:703
Kuhn H, Friederich U, Fiechter A (1979) Defined minimal medium for a thermophilic Bacillus sp. developed by a chemostat pulse and shift technique. Europ J Appl Microbiol Biotechnol 6:341–349
Kuhn HJ, Cometta S, Fiechter A (1980) Effects of growth temperature on maximal specific growth rate, yield, maintenance and death rate in glucose-limited continuous culture of the thermophilic Bacillus caldotenax. Europ J Appl Microbiol Biotechnol 10:303–315
Lawford HG, Haddock BA (1973) Respiration-driven proton translocation in Escherichia coli. Biochem J 136:217–220
McCarthy JEG, Ferguson SJ, Kell DB (1981) Estimation with an ionselective electrode of the membrane potential in cells of Paracoccus denitrificans from the uptake of BTPP+ during aerobic and anaerobic respiration. Biochem J 196:311–321
McFetters GA, Ulrich JT (1972) Effect of temperature on the respiration and cytochromes of an extreme thermophile. J Bacteriol 110:777–779
Mitchell P (1966) Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev 41:445–502
Mitchell P, Moyle J (1967) Respiration-driven proton translocation in rat liver mitochondria. Biochem J 105:1147–1162
Ratcliffe HD, Drozd JW, Bull AT (1979) Energy coupling in Rhizobium sp. Soc Microbiol Quart 6:72–73
Rosing J, Slater EC (1972) The value of ΔG0 for the hydrolysis of ATP. Biochim Biophys Acta 267:275–290
Rottenberg H (1979) Non-equilibrium thermodynamics of energy conversion in bioenergetics. Biochim Biophys Acta 549:225–253
Scholes PB, Mitchell P (1970) Respiration-driven proton translocation in Micrococcus denitrificans. J Bioenerget 1:309–323
Sone N, Ohyama T, Kagawa Y (1979) Thermostable single-band cytochrome oxidase. FEBS Lett 106:39–42
Sorgato MC, Ferguson SJ, Kell DB, John P (1978) The protonmotive force in bovine heart submitochondrial particles. Biochem J 174:237–256
Stouthamer AH, Bettenhaussen C (1975) Determination of the efficiency of oxidative phosphorylation in continuous cultures of Aerobacter aerogenes. Arch Microbiol 102:187–192
Willison JC, Haddock BA (1981) The efficiency of energy conservation in cytochrome c-deficient mutant of Paracoccus denitrificans. FEMS Microbiol Lett 10:53–57
Zilberstein D, Schuldinger S, Padan E (1979) Proton electrochemical gradient in Escherichia coli cells and its relation to the active transport of lactose. Biochemistry 18:669–673
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McKay, A., Quilter, J. & Jones, C.W. Energy conservation in the extreme thermophile Thermus thermophilus HB8. Arch. Microbiol. 131, 43–50 (1982). https://doi.org/10.1007/BF00451497
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00451497