Abstract
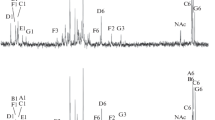
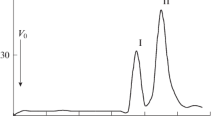
Lipopolysaccharides were obtained from three strains (PCC 7806, PCC 7820 and UV-017) of the waterbloom-forming cyanobacterium Microcystis aeruginosa. 3-Hydroxy fatty acids (3-OH-14:0, 3-OH-16:0, 3-OH-18:0) in addition to other fatty acids were identified in all three lipopolysaccharides. Glucosamine, the only amino sugar found, presumably represents the backbone amino sugar of the phosphate-free lipid A moiety. Heptoses were absent and 2-keto-3-deoxyoctonate was not detected in all three lipopolysaccharides. Strain-specificity was revealed in the complex composition of the polysaccharide moieties. Strains PCC 7806 and UV-017 were of the same chemotype, it differs from that of strain PCC 7820. Polysaccharides with strain-specific chemical compositions different from those of the respective lipopolysaccharides were obtained from each strain. The polysaccharides are likely to be ascribed to external cell envelope layers, their sugar specificity was in parallel with the O-chain chemotypes of the lipopolysaccharides of the three strains.
Similar content being viewed by others
Abbreviations
- GLC/MS:
-
combined gas-liquid chromatography/mass spectrometry
- KDO:
-
2-keto-3-deoxyoctonate
- PCC:
-
Pasteur Culture Collection
References
Amemiya Y, Nakayama O (1984) The chemical composition and metal adsorption capacity of the sheath materials isolated from Microcystis cyanobacteria. Jpn J Limnol 45:187–193
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Botes DP, Wessels PL, Kruger H, Runnegar MTC, Santikarn S, Smith R, Barna J, Williams D (1985) Structural studies on cyanoginosins-LR, YR, YA and YM, peptide toxins from Microcystis aeruginosa. J Chem Soc Perkn Trans I:2747–2748
Botes DP, Viljoen CC, Kruger H, Wessels PL, Williams D (1982) Configuration assignments of the amino acid residue and the presence of N-methyldehydroalanine in toxins from the bluegreen alga Microcystis aeruginosa. Toxicon 20:1037–1042
Brade H, Galanos C (1983) A method to detect 2-keto-3-deoxyoctonate and related compounds on pherograms and chromatograms. Anal Biochem 132:158–159
Carmichael WW, Mahmood NA (1984) Toxins from freshwater cyanobacteria. In: Ragelis EP (ed) Seafood toxins. American Chem Soc Symp Series 262, Wash. DC, pp 377–389
Codd GA (1984) Toxins of freshwater cyanobacteria. Microbiol Sci 1:48–52
Codd GA, Carmichael WW (1982) Toxicity of a clonal isolate of the cyanobacterium Microcystis aeruginosa from Great Britain. FEMS Microbiol Lett 13:409–411
Dierstein R, Kaiser I, Weckesser J (1988) Rapid determination of Microcystis aeruginosa toxins by reversed phase liquid chromatography. FEMS Microbiol Lett 49:143–147
Drews G, Weckesser J (1982) Fine structure and chemical composition of the cell envelopes. In: Carr NG, Whitton BA (eds) The biology of cyanobacteria. Blackwell Scientific Publications, Oxford, UK, pp 96–116
Evers D, Weckesser J, Jürgen UJ (1986) Chemical analysis on cell envelope polymers of the halophilic, phototrophic Rhodospirillum salexigens. Arch Microbiol 145:254–258
Geitler L (1925) Synoptische Darstellung der Cynaphyceen in morphologischer und systematischer Hinsicht. Beih Bot Cbl 41:163–294
Gentile J (1971) Blue-green and green algal toxins. In: Ajl SJ (eds) Microbial toxins, vol VII, Algal and fungal toxins. Academic Press, New York, pp 27–66
Gorham PR, Carmichael WW (1979) Phycotoxins from blue green algae. Pure and Applied Chemistry 52:165–174
Hughes EO, Gorham RP, Zehnder A (1958) Toxicity of an unialgal culture of Microcystis aeruginosa. Can J Microbiol 4:225–236
Jansson PE, Kenne L, Liedgren H, Lindberg B, Lunngren J (1976) A practical guide to the methylation analysis of carbohydrates. Chem Comm 8:1–74
Karlsson B, Vaara T, Loutnatmaa K, Gyllenberg H (1983) Three-dimensional structure of the regularly constructed surface layer from Synechocystis sp. strain CLII. J Bacteriol 156:1338–1343
Kessel M, Eloff J (1975) The ultrastructure and development of the colonial sheath of Microcystis marginata. Arch Microbiol 106:209–214
Kessel M (1978) The unique crystalline wall layer in the cyanobacterium Microcystis marginata. J Ultrastr Res 62:203–212
Krauss JH, Weckesser J, Mayer H (1988) Electrophoretic analysis of lipopolysaccharides of purple nonsulfur bacteria. Int J Syst Bacteriol 38:157–163
Kickhöfen B, Warth R (1968) Eine Trennkammer für die Hochspannungselektrophorese nach dem Michelschen Prinzip. J Chromatogr 33:558–560
Lowry OH, Roberts NR, Leiner KY, Wu ML, Farr AL (1954) The quantitative histochemistry of brain. J Biol Chem 207:1–17
Niedermeier W, Tomana M (1974) Gas chromatographic analysis of hexosamines in glycoproteins. Anal Biochem 57:363–368
Partridge SM (1949) Aniline hydrogen phthalate as a spraying reagent for chromatography of sugars. Nature 164:443
Raziuddin S, Siegelman HW, Tornabene TG (1983) Lipopolysaccharides of the cyanobacterium Microcystis aeruginosa. Eur J Biochem 137:333–336
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Roppel J, Mayer H, Weckesser J (1975) Identification of a 2,3-diamino-2,3-dideoxyhexose in the lipid A component of the lipopolysaccharides of Rhodopseudomonas viridis and Rhodopseudomonas palustris. Carbohydr Res 40:31–40
Ryhage R Stenhagen E (1960) Mass spectrometric studies. VI. Methyl esters of normal chain oxo-, hydroxy-, methyxy-and epoxy-acids. Arkiv Kemi 15:545–560
Sawardeker JS, Sloneker JH, Jeanes A (1967) Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Anal Chem 37:1602–1604
Schmidt W, Drews G, Weckesser J, Fromme I, Borowiak D (1980a) Characterization of the lipopolysaccharides from eight strains of the cyanobacterium Synechococcus Arch Microbiol 127:209–215
Schmidt W, Drews G, Weckesser J, Mayer H (1980b) Lipopolysaccharides in four strains of the unicellular cyanobacterium Synechocystis. Arch Microbiol 127:217–222
Sleytr UB, Messner P (1983) Crystalline surface layers of bacteria. Annu Rev Microbiol 37:311–319
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Sykora JL, Keleti G, Roche R, Volk DR, Kay GP, Burgess RA, Shapiro MA (1980) Endotoxins, algae and Limulus amoebocyte lysate test in driking water. Water Res 14:829–839
Warawdekar VS, Saslaw LD (1959) A sensitive method for the estimation of 2-deoxy sugars with the use of the thiobarbituric acid reaction. J Biol Chem 234:1945–1950
Weckesser J, Mayer H (1988) Different lipids A types in lipopolysaccharides of phototrophic and related non-phototrophic bacteria. FEMS Microbiol Lett 54:143–154
Weckesser J, Drews G, Mayer H (1979) Lipopolysaccharides of photosynthetic prokaryotes. Annu Rev Microbiol 33:215–239
Westphal O, Lüderitz O, Bister F (1952) Über die Extraktion von Bakterien mit Phenol/Wasser. Z Naturforsch 7b:148–155
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Martin, C., Codd, G.A., Siegelman, H.W. et al. Lipopolysaccharides and polysaccharides of the cell envelope of toxic Microcystis aeruginosa strains. Arch. Microbiol. 152, 90–94 (1989). https://doi.org/10.1007/BF00447017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00447017