Abstract
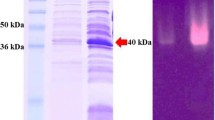
The gene encoding β-mannanase was cloned from alkalophilic Bacillus sp. AM-001 into Escherichia coli JM 101 by inserting HindIII-generated DNA fragments into the HindIII site of pUC19. A 2.0 kb XbaI-PstI fragment of the donor strain DNA was sufficient for β-mannanase synthesis. The amount of β-mannanase expressed in E. coli JM101 harboring pMAH3 (containing a 2.4 kb XbaI-HindIII fragment) was about 24% of the activity produced by the donor strain. E. coli JM101 harboring pMAH3 was found to produce two enzymatically active β-mannanases (A and B). These two β-mannanases were purified to electrophoretically homogenous states. The β-mannanase A had enzymatic properties similar to those of the β-mannanases M-I and M-II produced by alkalophilic Bacillus sp. AM-001, and the β-mannanase B resembled its β-mannanase M-III. In contrast to β-mannanase production in the donor strain, that in E. coli was not inducible. The NH2-terminal amino acid sequences from amino acid 1 (Asn) to 9 (Gln) of the three β-mannanases purified from alkalophilic Bacillus sp. AM-001 coincide with those from amino acid 4 (Asn) to 12 (Gln) of the two β-mannanases purified from E. coli transformant.
Similar content being viewed by others
References
Akino T, Nakamura N, Horikoshi K (1987) Production of β-mannosidase and β-mannanase by an alkalophilic Bacillus sp. Appl Microbiol Biotechnol 26:323–327
Akino T, Nakamura N, Horikoshi K (1988a) Characterization of three β-mannanases of an alkalophilic Bacillus sp. Agric Biol Chem 52:773–779
Akino T, Nakamura N, Horikoshi K (1988b) Characterization of β-mannosidase of an alkalophilic Bacillus sp. Agric Biol Chem 52:1459–1464
Araki T (1983) Purification and characterization of an endo-β-mannanase from Aeromonas sp. F-25. J Fac Agr Kyushu Univ 27:89–98
Bolivar E, Rodriguez RL, Greene PJ, Betlach MC, Heynecker HL, Boyer HW (1977) Construction and characterization of new cloning vehicles. II. A multi-purpose cloning system. Gene 2:95–113
Edman P, Henschen A (1975) Protein sequence determination. In: Needleman SB (ed) Sequence determination. Springer, Berlin Heidelberg New York, pp 232–279
Emi S, Fukumoto J, Yamamoto T (1972) Crystallization and some properties of mannanase. Agric Biol Chem 36:991–1001
Eriksson KE, Winnel M (1968) Purification and characterization of a fungal β-mannanase. Acta Chem Scand 22:1924–1934
Hashimoto Y, Fukumoto J (1969) Studies on the enzyme treatment of coffee beans. Part I. Purification of mannanase of Rhizopus niveus and its action on coffee mannan. Nippon Nogeikagaku Kaishi 43:317–322
Hussain M, Carlino A, Madonna MJ, Lampen JO (1985) Cloning and sequencing of the metallothioprotein β-lactamase II gene of Bacillus cereus 569/H in Escherichia coli. J Bacteriol 164:223–229
Kato C, Kudo T, Watanabe K, Horikoshi K (1983) Extracellular production of Bacillus penicillinase by Escherichia coli carrying pEAP2. Eur J Appl Microbiol Biotechnol 18:339–343
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 227:680–685
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning, a laboratory manual. Cold Spring Harbor, New York, Cold Spring Harbor Laboratory, pp 68–69
Norgard MV, Keem K, Monahan JJ (1978) Factors affecting the transformation of Escherichia coli strain χ 1776 by pBR322 plasmid DNA. Gene 3:279–292
Panbangred W, Kondo T, Negoro S, Shinmyo A, Okada H (1983) Molecular cloning of the genes for xylan degradation of Bacillus pumilus and their expression in Escherichia coli. Mol Gen Genet 192:335–341
Rigby PWJ, Dieckmann M, Rhodes C, Berg P (1977) Labeling deoxyribonucleic acid to high specific activity in vitro by nicktranslation with DNA polymerase I. J Mol Biol 113:237–251
Saito H, Miura K (1963) Preparation of transforming deoxyribonucleic acid by phenol treatment. Biochim Biophys Acta 72:619–629
Smith F, Srivastava HC (1959) Constitutional studies on the glucomannan of konjak flour. J Am Chem Soc 81:1715–1718
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Takahashi R, Kusakabe I, Kobayashi H, Murakami K, Maekawa A, Suzuki I (1984) Purification and some properties of mannanase from Streptomyces sp. Agric Biol Chem 48:2189–2195
Takeshita S, Sato M, Toba M, Masahashi W, Hashimoto T (1987) High-copy-number and low-copy-number plasmid vectors for lacZα-complementation and chloramphenicol or kanamycin-resistance selection. Gene 6:63–74
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Yanisch-Perron C, Viera J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mp18 and pUC19 vectors. Gene 33:103–119
Zama M, Kusakabe I, Murakami K (1985) Enzymatic preparation of crystalline mannose from copra mannan. Jap J Trop Agr 29:221–225
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Akino, T., Kato, C. & Horikoshi, K. The cloned β-mannanase gene from alkalophilic Bacillus sp. AM-001 produces two β-mannanases in Escherichia coli . Arch. Microbiol. 152, 10–15 (1989). https://doi.org/10.1007/BF00447004
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00447004