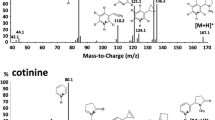
Abstract
A rapid and sensitive capillary gas-chromatographic method with nitrogen-sensitive detection is reported for the simultaneous analysis of nicotine and cotinine levels occurring in the plasma, saliva, and urine of regular tobacco smokers. The proposed assay has a linear output, has satisfactory accuracy over the range of concentrations of both amines encountered in active smokers, and has also been successful in the analysis of the urine samples of passive smokers. Its lower limit of sensitivity is 0.2 ng of nicotine and 0.5 ng of cotinine per ml of plasma or saliva or per 100 μl of urine.
The beneficial characteristics of the presented method were achieved by the combination of solid phase extraction of 0.1–1.0 ml of fluid specimens, capillary column gas chromatography with splitless injection and nitrogen sensitive detection, and the use of separate, structurally analogous compounds as internal standards for nicotine. The suitability of the assay is shown by plasma concentration-time curves of nicotine and cotinine in a steady smoker during a 24 hours period.
Similar content being viewed by others
References
Benowitz NL (1986) Ann. Rev. Med. 37: 21
Horning EC, Horning MG, Carroll DI, Stillwell RN & Dzidic I (1973) Life Sciences 13: 1331
Langone JJ, Gjika HB & VanVunakis H (1973) Biochemistry 12: 5025
Haines CFJr, Mahajan DK, Miljkovic D et al. (1976) Clin. Pharmacol. Ther. 16: 1083
Castro A, Monji N, Ali H, Malkus H, Eisenhart W, McKennis HJr & Bowman ER (1979) Clinica Chimica Acta 95: 473
Matsukura S, Sakamoto N, Seino Y et l. (1979) Clin. Pharmacol. Ther. 25: 555
Hill P & Marquardt H (1980) Clin. Pharmacol. Ther. 27: 652
Castro A, Monji N, Ali H, Yi JM, Bowman ER & McKennis HJr (1980) Eur. J. Bioch. 104: 331
Langone JJ & VanVunakis H (1982) Methods in Enzymology 84: 628
Greenberg R, Etzel R, Haley N & Loda H (1984) Am. J. Epidemiology 118: 435
Knight GJ, Wylie P, Holman MS & Haddow JE (1985) Clin. Chem. 31: 118
Hansel MC, Rowell FR, Landon J & Sidki AM (1986) Ann. Clin. Biochem. 23: 596
Watson ID (1977) J. Chromatogr. 143: 203
Maskarinec MP, Harvey RW & Caton JE (1978) J. Anal. Toxicol. 2: 124
Kyerematen GA, Damiano MD, Dvorchik BH & Vesell ES (1982) Clin. Pharm. Ther. 32: 769
Beckett AH & Triggs EJ (1966) Nature 211: 1415
Feyerabend C & Russell MAH (1980) Analyst 105: 998
Stehlik G, Kainzbauer J, Tausch H & Richter O (1982) J. Chromatogr. 232: 295
Blache D, Thevenon C, Ciavatti M & Renaud S (1984) Anal. Biochem. 143: 316
Hartvig P, Ahnfelt N-O, Hammarlund M & Vessman J (1979) J. Chromatogr. 173: 127
Isaac PF & Rand MJ (1972) Nature 236: 308
Hengen N & Hengen M (1978) Clin. Chem. 24: 50
Feyerabend C & Russell MAH (1979) J. Pharm. Pharmacol. 31: 73
Grubner O & First MW (1980) Anal. Chem. 52: 1755
Kogan MJ, Vereby K, Jaffee JH & Mule SJ (1981) J. Forens. Sci. 26: 6
Jacob PIII, Wilson M & Beowitz NL (1981) J. Chromatogr. 222: 61
Curvall M, Kazemi-Vala E & Enzell CR (1982) J. Chromatogr. 232: 283
Davis RA (1986) J. Chrom. Sci. 24: 134
Thompson JA, Ho M-S & Petersen DR (1982) J. Chromatogr. 231: 53
Falkman SE, Burrows IE, Lundgren RA & Page BFJ (1975) Analyst 100: 99
Dow J & Hall K (1978) J. Chromatogr. 153: 521
Gruenke LD, Beelen TC, Cymerman Craig J & Petrakis NL (1979) Anal. Biochem. 94: 411
Daenens P, Laruelle L, Callewaert K, DeSchepper P, Galeazzi R & VanRossum J (1985) J. Chromatogr. 342: 79
Freeman RR, ed. (1981) High Resolution Gas Chromatography, 2nd edition (pp 59–62) Hewlett-Packard Company, Palo Alto, California, USA
Benowitz NL, Jacob PIII, Jones RT & Rosenberg J (1984) J. Pharmacol. Exp. Ther. 221: 368
Benowitz NL, Kuyt F, Jacob PIII, Jones RT & Osman AL (1983) Clin. Pharmacol. Ther. 34: 604
VanHorne KC, ed. (1985) Sorbent Extraction Technology, 1st edition. Analytichem International, Inc., Harbor City, California, USA
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Teeuwen, H.W.A., Aalders, R.J.W. & Van Rossum, J.M. Simultaneous estimation of nicotine and cotinine levels in biological fluids using high-resolution capillary-column gas chromatography combined with solid phase extraction work-up. Molecular Biology Reports 13, 165–175 (1988). https://doi.org/10.1007/BF00444313
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00444313