Abstract
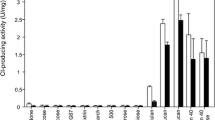
Cytoplasmic membranes from mycelium or protoplasts of Saprolegnia monoica (a cellulosic cell-wall fungus) were separated by continuous sucrose-density-gradient centrifugation. Glucan synthases assayed at low (micromolar uridine 5′-diphosphate (UDP) glucose for β-1-4-glucan synthase) and high (millimolar UDP glucose for β-1-3-glucan synthase) substrate concentrations were associated with membranes exhibiting vanadate-sensitive, oligomycin-insensitive ATPase and equilibrating at density 1.16 g cm-3. Synthase activities were also bound to membranes of lower density (1.10 and 1.145 g cm-3). Plasma membranes were stabilized by coating protoplasts with concanavalin A. After lysis of the protoplasts, plasma membranes recovered by low centrifugal forces were isolated in continuous isopycinic gradients. Both synthase activities peaked with [3H]concanavalin A and Na-vanadate ATPase indicating that the synthetases are located at the plasma membrane. Treatments of intact protoplasts with cold glutaraldehyde or proteases before disruption lead to a diminution of glucan-synthase activities indicating that at least part of the enzymes of plasma membrane face the outside of the cell.
Similar content being viewed by others
Abbreviations
- ConA:
-
concanavalin A
- ER:
-
endoplasmic reticulum
- GSI:
-
β-1,4-glucan synthase
- GSH:
-
β-1,3-glucan synthase
- UDP:
-
uridine 5′-diphosphate
References
Boss, W.F., Ruesink, A.W. (1979) Isolation and characterization of Concanavalin A-labeled plasma membranes of carrot protoplasts. Plant Physiol. 64, 1005–1011
Bowles, D.J., Quail, P.H., Morré, D.J., Hartmann, G.C. (1979) Use of markers in plant cell fractionation. In: Plant organelles, pp. 207–223, Reid, C., ed. Ellis Horwood, Chichester
Bowman, E.J., Bowman, B.J., Slayman, C.W. (1981) Isolation and characterization of plasma membranes from wild type Neurospora crassa. J. Biol. Chem. 256, 12336–12342
Duran, A., Bowers, B., Cabib, E. (1975) Chitin synthetase zymogen is attached to the yeast plasma membrane. Proc. Natl. Acad. Sci. USA 72, 3952–3955
Fèvre, M. (1979a) Glucanases, glucan synthases and wall growth in Saprolegnia monoica. In: Fungal walls and hyphal growth, pp. 225–263, Burnett, J.H., Trinci, A.P.J. eds. Cambridge University Press, Cambridge
Fèvre, M. (1979b) Digitonin solubilization and protease stimulation of β glucan synthetases of Saprolegnia. Z. Pflanzenphysiol. 95, 129–140
Fèvre, M., Dumas, C. (1977) β glucan synthetases from Saprolegnia monoica. J. Gen. Microbiol. 103, 297–306
Fèvre, M., Rougier, M. (1981) β 1–3 and β 1–4 glucan synthesis by membrane fractions from the fungus Saprolegnia. Planta 151, 232–241
Gaugy, D., Fèvre, M. (1982) Protoplast production from Saprolegnia monoica. Microbios 34, 89–98
Giddings, Th., Brower, D.L., Staehelin, L.A. (1980) Visualization of particles complexes in the plasma membrane of Micrasterias denticulata associated with the formation of cellulose fibrils in primary and secondary cell walls. J. Cell Biol. 84, 327–339
Goffeau, A., Slayman, C.W. (1981) The proton-translocating ATPase of the fungal plasma membrane. Biochim. Biophys. Acta 639, 197–223
Hendriks, T. (1978) The distribution of glucan synthetase in maize coleoptiles: a comparison with K-ATPase. Plant Sci. Lett. 11, 261–274
Machlis, L. (1953) Growth and nutrition of water molds in the subgenus Euallomyces. II. Optimal composition of the minimal medium. Am. J. Bot. 40, 449–460
Montezinos, D. (1982) The role of the plasma membrane in cellulose microfibril assembly. In: The cytoskeleton in plant growth and development, pp. 147–162, Lloyd, C.W., ed. Academic Press, London New York
Mueller, S.C. (1982) Cellulose-microfibril assembly and orientation in higher plant cells with particular reference to seedlings of Zea mays. In: Cellulose and other natural polymer systems. Biogenesis, structure and degradation, pp. 87–104, Brown Jr., R.M., ed. Plenum Press, New York London
Mueller, S.C., Brown Jr., R.M. (1980) Evidence for an intramembrane component associated with a cellulose microfibril-synthesizing complex in higher plants. J. Cell Biol. 84, 315–326
Phillippi, M.L., Parish, R.W. (1981) Changes in glucan synthetase activity and plasma membrane proteins during encystment of the cellular slime mold Polysphondylium pallidum. Planta 152, 59–69
Pierce, W.S., Hendrix, D.L. (1979) Utilization of enzyme markers to determine the location of plasma membrane from Pisum epicotyls on sucrose gradients. Planta 146, 161–169
Quail, P.H. (1979) Plant cell fractionation. Annu. Rev. Plant Physiol. 30, 425–484
Santos, E., Villanueva, J.R., Sentandreu, R. (1978) The plasma membrane of Saccharomyces cerevisiae: isolation and some properties. Biochim. Biophys. Acta 508, 39–54
Scarborough, G.A. (1975) Isolation and characterization of Neurospora crassa plasma membranes. J. Biol. Chem. 250, 1106–1111
Scarborough, G.A. (1977) Properties of the Neurospora crassa plasma membrane ATPase. Arch. Biochem. Biophys. 180, 384–393
Shematek, E.M., Braatz, J.A., Cabib, E. (1980) Biosynthesis of the yeast cell wall. I. Preparation and properties of β (1–3) glucan synthetase. J. Biol. Chem. 255, 888–894
Shore, G., Maclachlan, G.A. (1975) The site of cellulose synthesis. Hormone treatment alters the intracellular location of alkali-insoluble β 1–4 glucan (cellulose) synthetase activities. J. Cell Biol. 64, 557–571
Tolbert, N.E. (1974) Isolation of subcellular organelles of metabolism on on isopycnic sucrose gradients. Methods Enzymol. 31, 734–746
Van Der Woude, N.J., Lembi, C.A., Morré, D.J., Kindinger, J.I., Ordin, L. (1974) B glucan synthetases of plasma membrane and golgi apparatus from onion stem. Plant Physiol. 54, 333–340
Wang, M.L., Bartnicki-Garcia, S. (1976) Synthesis of β 1–3 glucan microfibril by a cell-free extract of Phytophthora cinamomi. Arch. Biochem. Biophys. 175, 351–354
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Girard, V., Fèvre, M. β-1-4-and β-1-3-glucan synthases are associated with the plasma membrane of the fungus Saprolegnia . Planta 160, 400–406 (1984). https://doi.org/10.1007/BF00429755
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00429755