Summary
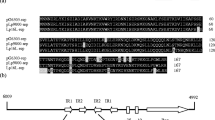
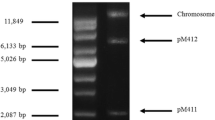
pMV158 is a 5.4 kb broad host range multicopy plasmid specifying tetracycline resistance. This plasmid and two of its derivatives, pLS1 and pLS5, are stably mantained and express their genetic information in gram-positive and gram-negative hosts. The in vitro replication of plasmid pMV158 and its derivatives was studied in extracts prepared from plasmid-free Escherichia coli cells and the replicative characteristics of the streptococcal plasmids were compared to those of the E. coli replicons, ColE1 and the mini-R1 derivative pKN182. The optimal replicative activity of the E. coli extracts was found at a cellular phase of growth that corresponded to 2 g wet weight of cells per litre. Maximal synthesis of streptococcal plasmid DNA occurred after 90 min of incubation and at a temperature of 30° C. The optimal concentration of template DNA was 40 μg/ml. Higher plasmid DNA concentrations resulted in a decrease in the incorporation of dTMP, indicating that competition of specific replication factor(s) for functional plasmid origins may occur. In vitro replication of plasmid pMV158 and its serivatives required the host RNA polymerase and de novo protein synthesis. The final products of the streptococcal plasmid DNAs replicated in the E. coli in vitro system were monomeric supercoiled DNA forms that had completed at least one round of replication, although a set of putative replicative intermediates could also be found. The results suggest that a specific plasmid-encoded factor is needed for the replication of the streptococcal plasmids.
Similar content being viewed by others
References
Bachmann BJ (1972) Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev 36:525–557
Bagdasarian M, Lurz R, Rückert B, Franklin RCH, Bagdasarian MM, Frey J, Timmis KN (1981) Specific purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 16:237–247
Ballester S, López P, Alonso JC, Espinosa M, Lacks SA (1986) Selective advantage of deletions enhancing chloramphenicol acetyltransferase gene expression in Streptococcus pneumoniae plasmids. Gene 41:153–163
Burdett V (1980) Identification of tetracycline resistant R-plasmids in Streptococcus agalactiae (group B). Antimicrob Agents Chemother 18:753–760
Castagnoli L, Lacatena RM, Cesareni G (1985) Analysis of dominant copy number mutants of the plasmid pMB1. Nucleic Acids Res 13:5353–5367
Clewell DB (1981) Plasmids drug resistance and gene transfer in the genus Streptococcus. Microbiol Rev 45:409–436
Clewell DB, Helinski DR (1969) Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an open circular DNA form. Proc Natl Acad Sci USA 62:1159–1166
Diaz R, Ortega S (1984) Initiation of plasmid R1 replication in vitro is independent of transcription by host RNA polymerase. Nucleic Acids Res 12:5175–5191
Diaz R, Staudenbauer WL (1982a) Origin and direction of mini-R1 plasmid DNA replication in cell extracts of Escherichia coli. J Bacteriol 150:1077–1084
Diaz R, Staudenbauer WL (1982b) Replication of the broad host range plasmid RSF1010 in cell-free extracts of Escherichia coli and Pseudomonas aeruginosa. Nucleic Acids Res 10:4687–4702
Diaz R, Nordström K, Staudenbauer WL (1981) Plasmid R1 DNA replication dependent on protein synthesis in cell-free extracts of Escherichia coli. Nature 289:326–328
Ehrlich SD (1977) Replication and expression of plasmids from Staphylococcus aureus in Bacillus subtilis. Proc Natl Acad Sci USA 74:1680–1682
Espinosa M, López P, Pérez Ureña MT, Lacks SA (1982) Interspecific plasmid transfer between Streptococcus pneumoniae and Bacillus subtilis. Mol Gen Genet 188:195–201
Goze A, Ehrlich SD (1980) Replication of plasmids from Staphylococcus aureus in Escherichia coli. Proc Natl Acad Sci USA 77:7333–7337
Humphreys GO, Willshaw GA, Anderson ES (1975) A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta 383:457–463
Inuzuka M, Helinski DR (1978) Requirement of a plasmid-encoded protein for replication in vitro of plasmid R6K. Proc Natl Acad Sci USA 75:5381–5385
Khan SA, Carleton SM, Novick RP (1981) Replication of plasmid pT181 DNA in vitro: Requirement for a plasmid-encoded product. Proc Natl Acad Sci USA 78:4902–4906
Koepsel RR, Murray RW, Rosenblum WD, Khan SA (1985a) Purification of pT181-encoded RepC protein required for the initiation of plasmid replication. J Biol Chem 260:8571–8577
Koepsel RR, Murray RW, Rosenblum WD, Khan SA (1985b) The replication initiator protein of plasmid pT181 has sequence-specific endonuclease and topoisomerase-like activities. Proc Natl Acad Sci USA 82:6845–6849
Kok J, van der Vossen JMB, Venema G (1984) Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl Environ Microbiol 48:726–731
Lacks SA (1966) Integration efficiency and genetic recombination in pneumococcal transformation. Genetics 53:207–235
Lacks SA, López P, Greenberg B, Espinosa M (1986) Identification and analysis of genes for tetracycline resistance and replication functions in the broad-host-range plasmid pLS1. J Mol Biol 192:753
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Molin S, Nordström K (1980) Control of plasmid R1 replication: Functions involved in replication, copy number control, incompatibility, and switch-off of replication. J Bacteriol 141:111–120
Saunders CW, Guild WR (1980) Properties and transforming activities of two plasmids in Streptococcus pneumoniae. Mol Gen Genet 180:573–578
Scott JR (1984) Regulation of plasmid replication. Microbiol Rev 48:1–23
Stassi DL, López P, Espinosa M, Lacks SA (1981) Cloning of chromosomal genes in Streptococcus pneumoniae. Proc Natl Acad Sci USA 78:7028–7032
Staudenbauer WL (1976) Replication of small plasmids in extracts of Escherichia coli. Mol Gen Genet 145:273–280
Staudenbauer WL (1984) Plasmid DNA replication in vitro. In: Pühler A, Timmis KN (eds) Advances molecular genetics. Springer, Berlin, Heidelberg, New York, Tokyo, pp 325–337
te Riele H, Michel B, Ehrlich SD (1986) Are single-stranded circles intermediates in plasmid DNA replication? EMBO J 5:631–637
Author information
Authors and Affiliations
Additional information
Communicated by H. Böhme
Rights and permissions
About this article
Cite this article
del Solar, G., Diaz, R. & Espinosa, M. Replication of the streptococcal plasmid pMV158 and derivatives in cell-free extracts of Escherichia coli . Mol Gen Genet 206, 428–435 (1987). https://doi.org/10.1007/BF00428882
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00428882