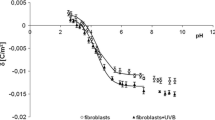
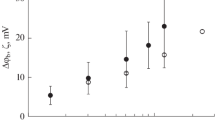
Summary
Electron spin resonance spectroscopy using the spin probe (5-, 12-and 16-deoxylstearic acid) was employed to analyze the changes in membrane fluidity in B-16 melanoma cells following UV-B exposure. The UV exposure resulted in the immediate accumulation of lipid peroxide, being accompanied by a change in membrane fluidity. The 12-DSA is the most sensitive to the changes in membrane organization caused by UV light. Na+,K+-ATPase activity was regulated by a change in membrane fluidity. Following UV exposure, the release of the prelabeled arachidonic acid from the cells was observed immediately. Ca2+-dependent calmodulin-dependent phospolipase A2-like activity was involved in the UV-stimulated arachidonic acid release from phospholipid.
Similar content being viewed by others
References
Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20:470–475
Bartlett GR (1959) Phosphorus assay in column chromatography. J Biol Chem 234:466–468
Berridge M (1985) The molecular basis of communication within the cell. Sci Am 253:142–152
Bruch RC, Thayer WS (1983) Differential effect of lipid peroxidation on membrane fluidity as determined by electron spin resonance probes. Biochim Biophys Acta 733:216–222
Curtis MT, Gilfor D, Farber JL (1984) Lipid peroxidation increases the molecular order of mitochondria. Arch Biochem Biophys 235:644–649
Fitzpatrick TB, Pathak MA, Harber LC et al. (eds) (1974) Sun light and man (1st edn) University of Tokyo Press, Tokyo
Folch J, Lees M, Sloane-Stanley GHA (1957) A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem 226:497–507
Gaffney BJ (1975) Fatty acid chain flexibility in the membrane of normal and transformed fibroblasts. Proc Natl Acad Sci USA 72:664–668
Hill S, Bleehen SS, MacNeil S (1986) An investigation of the intracellular messenger systems involved in melanogenesis. 17th Meeting of the European Society for Dermatological Research, 29 March to 1 April Amsterdam
Ito T, Ohnishi S (1974) Ca2+-induced lateral phase separations in phosphatidic acid-phosphatidylcholine membranes. Biochim Biophys Acta 352:29–37
Keeffe EB, Scharschmidt BF, Blankenship NM, Ockner RF (1979) Studies of relationships among bile flow, liver plasma membrane NaK-ATPase, and membrane microviscosity in the rat. J Clin Invest 64:1590–1598
Kimelberg HK, Papahadjopoulos D (1974) Effects of phospholipid acyl chain fluidity, phase transitions, and cholesterol on (Na++K+)-stimulated adenosine triphosphatase. J Biol Chem 249:1071–1088
Liu MS, Ghosh S, Yang Y (1983) Change in membrane lipid fluidity induced by phospholipase A2 activity. Life Sci 33:1995–2002
Ogura R, Sakanashi T, Nagata O, Sugiyama M, Kajiyama K, Nakagawa T, Shin G, Hidaka T (1987) Assay for lipid peroxide content in mitochondria by the thiobarbituric acid reaction. Kurume Med J 34:53–58
Sakanashi T, Sugiyama M, Suematsu T, Ogura R, Hidaka T, Nakagawa T (1989) UV exposure alters membrane lipid compositions and cell membrane fluidity of intact cultured B-16 melanoma cells. Kurume Med J 35 (in press)
Schimmel SD, Kent C, Bischoff R, Vagelos PR (1973) Plasma membrane from cultured muscle cells: isolation procedure and separation of putative plasma-membrane marker enzymes. Proc Natl Acad Sci USA 70:3195–3199
Sevanian A, Petkova D, Kovanok K (1986) Rat liver microsomal phospholipase A2 and membrane fluidity. Int J Biochem 18:659–663
Suematsu T, Hidaka T, Sakanashi T, Sugiyama M, Ogura R (1989) Effect of UV-B irradiation on release of arachidonic acid from B-16 melanoma cells. Prostaglandins Leukotrienes Essential Fatty Acids (in press)
Sugiyama M, Sakanashi T, Suematsu T, Murakata Y, Ogura R (1984) Measurement of membrane fluidity of B-16 melanoma culture cell with ESR spin label method. J Kurume Med Assoc 47:1301–1308
Tanaka T, Sakanashi T, Kaneko N, Ogura R (1986) Spin labeling study on membrane fluidity of epidermal cell (Cow snout epidermis). J Invest Dermatol 747:745–747
Tanaka T, Hidaka T, Ogura R, Sugiyama M (1987) Changes of membrane fluidity and Na+,K+-ATPase activity during cellular differentiation in the guinea pig epidermis. Arch Dermatol Res 761:1–4
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ogura, R., Sugiyama, M., Sakanashi, T. et al. Membrane responses of B-16 melanoma cells to single exposure to ultraviolet light. Arch Dermatol Res 280, 481–486 (1989). https://doi.org/10.1007/BF00427661
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00427661