Abstract
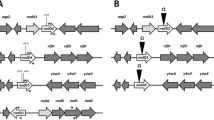
We report the genetic and biochemical analysis of Rhizobium meliloti mutants defective in symbiotic nitrogen fixation (Fix−) and “respiratory” nitrate reduction (Rnr−). The mutations were mapped close to the ade-1 and cys-46 chromosomal markers and the mutated locus proved to be identical to the previously described fix-14 locus. By directed Tn5 mutagenesis, a 4.5 kb segment of the chromosome was delimited in which all mutations resulted in Rnr− and Fix− phenotypes. Nucleotide sequence analysis of this region revealed the presence of four open reading frames coding for integral membrane and membrane-anchored proteins. Biochemical analysis of the mutants showed that the four proteins were necessary for the biogenesis of all cellular c-type cytochromes. In agreement with the nomenclature proposed for rhizobial genes involved in the formation of c-type cytochromes, the four genes were designated cycH, cycJ, cycK, and cycL, respectively. The predicted protein product of cycH exhibited a high degree of similarity to the Bradyrhizobium japonicum counterpart, while CycK and CycL shared more than 50% amino acid sequence identity with the Rhodobacter capsulatus Ccll and Cc12 proteins, respectively. cycJ encodes a novel membrane anchored protein of 150 amino acids. We suggest that this gene cluster codes for (parts of) a multi-subunit cytochrome c haem lyase. Moreover, our results indicate that in R. meliloti c-type cytochromes are required for respiratory nitrate reduction ex planta, as well as for symbiotic nitrogen fixation in root nodules.
Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local aligment tool. J Mol Biol 215:403–410
Appleby CA (1969) Electron transport systems of Rhizobium japonicum. II. Rhizobium hemoglobin, cytochromes and oxidases in free-living (cultured) cells. Biochim Biophys Acta 172:88–105
Appleby CA (1984) Leghemoglobin and Rhizobium respiration. Annu Rev Plant Physiol 35:443–478
Banfalvi Z, Kondorosi E, Kondorosi A (1985) Rhizobium meliloti carries two megaplasmids. Plasmid 13:129–138
Batut J, Daveran-Mingot M-L, David M, Jacobs J, Garnerone AM, Kahn D (1989) fixK, a gene homologous with fnr and crp from Escherichia coli, regulates nitrogen fixation genes both positively and negatively in Rhizobium meliloti. EMBO J 8:1279–1286
Beckman DL, Trawick DR, Kranz G (1992) Bacterial cytochrome c biogenesis. Genes Dev 6:268–283
Blattner FR, Burland V, Plunkett G, Sofia HJ, Daniels DL (1993) Analysis of the Escherichia coli genome. IV. DNA sequence of the region from 89.2 to 92.8 minutes. Nucleic Acids Res 21:5408–5417
Bott M, Bolliger M, Hennecke H (1990) Genetic analysis of the cytochrome c-aa 3 branch of the Bradyrhzobium japonicum respiratory chain. Mol Microbiol 4:2147–2157
Bott M, Ritz D, Hennecke H (1991) The Bradyrhizobium japonicum cycM gene encodes a membrane anchored homolog of mitochondrial cytochrome c. J Bacteriol 173:6766–6772
Brewin NJ, Beringer JE, Johnson AWB (1980) Plasmid mediated transfer of host-range specificity between two strains of Rhizobium leguminosarum. J Gen Microbiol 120:413–420
Creusot F, Verdiere J, Gaisne M, Slonimski PP (1988) CYP1 (HAP1) regulator of oxygen-dependent gene expression in yeast. J Mol Biol 204:263–276
Daldal F, Cheng S, Applebaum J, Davidson E, Prince RC (1986) Cytochrome c 2 is not essential for photosynthetic growth of Rhodobacter capsulata. Proc Natl Acad Sci USA 83:2012–2016
Darwin A, Hussain H, Griffiths L, Grove J, Sambongi Y, Busby S, Cole J (1993) Regulation and sequence of the structural gene for cytochrome c ss2 from Escherichia coli: not a hexahaem but a 50 kDa tetrahaem nitrite reductase. Mol Microbiol 9:1255–1265
Devereux J, Haberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12:387–395
Dumont ME, Ernst JF, Hampsey DM, Sherman F (1987) Identification and sequence of the gene encoding cytochrome c heme lyase in the yeast Saccharomyces cerevisiae. EMBO J 6:235–241
Eckhardt T (1978) A rapid method for the identification of plasmid deoxyribonucleic acid in bacteria. Plasmid 1:584–588
Eisenberg D, Schwarz E, Komaromy M, Wall R (1984) Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol 179:125–142
Forrai T, Vincze E, Banfalvi Z, Kiss GB, Randhawa A, Kondorosi A (1983) Localization of symbiotic mutations in Rhizobium meliloti. J Bacteriol 153:635–643
Francis RT, Becker RR (1984) Specific indication of hemoproteins in polyacrylamide gels using a double-staining process. Anal Biochem 136:509–514
Gonzales DH, Neupert W (1990) Biogenesis of mitochondrial ctype cytochromes. J Bioenerg Biomembr 22:753–768
Hussain H, Grove J, Griffiths L, Busby S, Cole J (1994) A sevengene operon essential for formate-dependent nitrite reduction to ammonia by enteric bacteria. Mol Microbiol 12:153–163
Kiss GB, Vincze E, Kálmán Z, Forrai T, Kondorosi A (1979) Genetic and biochemical analysis of mutants affected in nitrate reduction in Rhizobium meliloti. J Gen Microbiol 113:105–118
Knauf VC, Nester EW (1982) Wide host-range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid 8:45–54
Kondorosi A, Barabás I, Sváb Z, Orosz L, Sik T, Hotchkiss RD (1973) Evidence for common genetic determinants of nitrogenase and nitrate reductase in Rhizobium meliloti. Nature, New Biol 246:153–154
Kondorosi A, Kiss GB, Forrai T, Vincze E, Banfalvi Z (1977) Circular linkage map of Rhizobium meliloti chromosome. Nature 264:525–527
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Layzell DB, Hunt S, Moloney AHM, Fernando SM, del Castillo LD (1990) Physiological, metabolic and developmental implications of O2 regulation in legume nodules. In: Gresshoff PM, Roth LE, Stacey G, Newton WE (eds) Nitrogen fixation: achievements and objectives. Chapman and Hall, New York London, pp 21–32
Loferer H, Hennecke H (1994) Protein disulphide oxidoreductases in bacteria. Trends Biochem Sci 19: 169–171
Nicholson DW, Stuart RA, Neupert W (1989) Biogenesis of cytochrome c 1. J Biol Chem 264:10156–10168
Oda K, Yamato K, Ohta E, Nakamura Y, Takemura M, Nozato N, Akashi K, Kanegae T, Ogura Y, Kohchi T, Ohyama K (1992) Gene organization deduced from the complete sequence of liverwort Marchantia polymorpha mitochondrial DNA. J Mol Biol 223:1–7
Ohyama K, Fukuzawa H, Kohchi T, Shirai H, Sano T, Sano S, Umesono S, Shiki Y, Tkeuchi M, Chang Z, Aota S, Inokuchi H, Ozeki H (1986) Chloroplast gene organization deduced from the complete sequence of liverwort Marchantia polymorpha chloroplast DNA. Nature 322:572–574
Pearson WR, Lipman DJ (1988) Improved tools for biological sequence analysis. Proc Natl Acad Sci USA 85:2444–2448
Pettigrew GW, Moore GW (1987) Cytochromes c. Springer, Berlin, pp 160–179
Pfeifer K, Kim K-S, Kogan S, Guarente L (1989) Functional dissection and sequence of yeast HAP1 activator. Cell 56: 291–301
Preisig O, Anthamatten D, Hennecke H (1993) Genes for microaerobically induced oxidase complex in Bradyrhizobium japonicum are essential for a nitrogen-fixing endosymbiosis. Proc Natl Acad Sci USA 90:3309–3313
Putnoky P, Kondorosi A (1986) Two gene cluster of Rhizobium meliloti code for early essential nodulation functions and a third influences nodulation efficiency. J Bacteriol 167:881–887
Putnoky P, Grosskopf E, Ha DTC, Kiss GB, Kondorosi A (1988) Rhizobium fix genes mediate at least two communication steps in symbiotic nodule development. J Cell Biol 106:597–607
Putnoky P, Petrovics G, Kereszt A, Grosskopf E, Ha DTC, Bdnfalvi Z, Kondorosi A (1990) Rhizobium meliloti lipopolysaccharide and exopolysaccharide can have the same function in the plant-bacterium interaction. J Bacteriol 172:5450–5458
Ramseier TM, Winteler HV, Hennecke H (1991) Discovery and sequence analysis of bacterial genes involved in the biogenesis of c-type cytochromes. J Biol Chem 266:7793–7803
Rao MJK, Argos P (1986) A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta 869:197–214
Ritz D, Bott M, Hennecke H (1993) Formation of several bacterial c-type cytochromes requires a novel membrane-anchored protein that faces the periplasm. Mol Microbiol 9:729–740
Ritz D, Thöny-Meyer L, Hennecke H (1995) The cycHJKL gene cluster plays an essential role in the biogenesis of c-type cytochromes in Bradyrhizobium japonicum. Mol Gen Genet, in press
Rℏrig H, Schmidt J, Wieneke U, Kondorosi E, Barlier I, Schell J, John M (1994) Biosynthesis of lipooligosaccharide nodulation factors: Rhizobium NodA protein involved in N-acylation of the chitooligosaccharide backbone. Proc Natl Acad Sci USA 91:3122–3126
Ruvkun GB, Ausubel M (1981) A general method for site-directed mutagenesis in prokaryotes. Nature 289:85–88
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning — a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schuster W, Combettes B, Flieger K, Brennicke A (1993) A plant mitochondrial gene encodes a protein involved in cytochrome c biogenesis. Mol Gen Genet 239:49–57
Sik T, Barabas I (1977) The correlation of nitrate reductase and nitrogenase in Rhizobium symbiosis. In: Newton WE, Postgate JR, Rodriguez-Barrueco C (eds) Recent developments in nitrogen fixation. Academic Press, London, pp 365–373
Soberón M, Aguilar GR, Sánchez F (1993) Rhizobium phaseoli cytochrome c-deficient mutant induces empty nodules on Phaseolus vulgaris L. Mol Microbiol 8:159–166
Thöny-Meyer L, Stax D, Hennecke H (1989) An unusual gene cluster for the cytochrome bc 1 complex in Bradyrhizobium japonicum and its requirement for effective root nodule symbiosis. Cell 57:683–697
Thöny-Meyer L, Ritz D, Hennecke H (1994) Cytochrome c biogenesis in bacteria: a possible pathway begins to emerge. Mol Microbiol 12:1–9
Török I, Kondorosi E, Stepkowski T, Posfai J, Kondorosi A (1984) Nucleotide sequence of Rhizobium meliloti nodulation genes. Nucleic Acids Res 12:9509–9524
Von Heijne G (1986) A new method for predicting signal sequence cleavage sites. Nucleic Acids Res 14:4683–4690
Wieseler B, Schiltz E, Müller M (1992) Identification and solubilization of a signal peptidase from the phototrophic bacterium Rhodobacter capsulatus. FEBS Lett 298:273–276
Witty JF, Minchin FR (1990) Oxygen diffusion in the legume root nodule. In: Gresshoff PM, Roth LE, Stacey G, Newton WE (eds) Nitrogen fixation: achievements and objectives. Chapman and Hall, New York London, pp 285–292
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mp18 and pUC19 vectors. Gene 33:103–119
Author information
Authors and Affiliations
Additional information
Communicated by J. Lengeler
Rights and permissions
About this article
Cite this article
Kereszt, A., Slaska-Kiss, K., Putnoky, P. et al. The cycHJKL genes of Rhizobium meliloti involved in cytochrome c biogenesis are required for “respiratory” nitrate reduction ex planta and for nitrogen fixation during symbiosis. Molec. Gen. Genet. 247, 39–47 (1995). https://doi.org/10.1007/BF00425819
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00425819