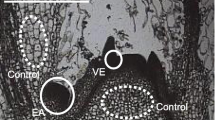
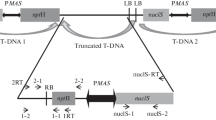
Summary
Transformed shoots regenerating from Crown gall tissues were produced by in vitro infection of regenerating tobacco protoplasts with Agrobacterium tumefaciens. As these shoots were abnormal in their growth and were unable to form roots, they were grafted onto normal tobacco, resulting in the formation of flowering shoots. Sexual crosses between different grafts and selfing produced two different types of seedlings. Some developed into normal, untransformed plants whereas others failed to develop a root system and remained very small. The abnormal seedlings were shown to consist of transformed cells since they expressed the opine marker. The suppression of root formation and the production of opines were 100 percent linked. The analysis of the progeny indicated that the shoots derived from these tumour lines were hemizygous for the T-DNA linked genes and expressed these genes as a dominant locus. The opine-positive shoots were shown to contain a deleted T-DNA and also some T-DNA specific transcripts: the transcript for octopine synthase or LpDH and two other transcripts, one of which is known to be involved in the suppression of root formation. The same transcripts could also be detected in the sexual progeny. These observations confirm that some active tumour-controlling genes can be sexually transmitted and do not drastically interfere with seed development, germination and early shoot formation. The formation of roots by these seedlings is however severely suppressed.
Similar content being viewed by others
Abbreviations
- LpDH:
-
Lysopine Dehydrogenase
- NpDH:
-
Nopaline Dehydrogenase
References
Akiyoshi DE, Morris RO, Hinz R, Mischke BS, Kosuge T, Garfinkel DJ, Gordon MP, Nester EW (1983) Cytokinin-auxin balance in crown gall tumors is regulated by specific loci in the T-DNA. Proc Natl Acad Sci USA 80:407–411
Amasino RM, Miller CO (1983) Effect of temperature on the morphology and cytokinin levels of tobacco crown gall teratoma tissues. Plant Sci Lett 28:245–253
Barton KA, Binns AN, Matzke AJM, Chilton M-D (1983) Regeneration of intact tobacco plants containing full length copies of genetically engineered T-DNA and transmission of T-DNA to R1 progeny. Cell 32:1033–1043
Bedbrook J (1981) A plant nuclear DNA preparation procedure. Plant Mol Biol Newslett, vol II, 24
Binns AN, Sciaky D, Wood HW (1982) Variation in hormone autonomy and regenerative potential of cells transformed by strain A66 of Agrobacterium tumefaciens. Cell 31:605–612
Braun AC(1959) A demonstration of the recovery of the crown-gall tumor cell with the use of complex tumors of single-cell origin. Proc Natl Acad Sci USA 45:932–938
Braun AC, White PR (1943) Bacteriological sterility of tissues derived from secondary crown gall tumors. Phytopathol 33:85–100
Braun AC, Wood HN (1976) Suppression of the neoplastic state with the acquisition of specialized functions in cells, tissues, and organs of crown gall teratomas of tobacco. Proc Natl Acad Sci USA 73:496–500
Chilton M-D, Drummond MH, Merlo DJ, Sciaky D, Montoya AL, Gordon MP, Nester EW (1977) Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell 11:263–271
Chilton M-D, Saiki RK, Yadav N, Gordon MP, Quetier F (1980) T-DNA from Agrobacterium Ti plasmid is in the nuclear DNA fraction of crown gall tumor cells. Proc Natl Acad Sci USA 77:4060–4064
De Beuckeleer M, Lemmers M, De Vos, G, Willmitzer L, Van Montagu M, Schell J (1981) Further insight on the transferred-DNA of octopine crown gall. Mol Gen Genet 183:283–288
De Greve H, Leemans J, Hernalsteens J-P, Thia-Toong L, De Beuckeleer M, Willmitzer L, Otten L, Van Montagu M, Schell J (1982) Regeneration of normal and fertile plants that express octopine synthase, from tobacco crown galls after deletion of tumor-controlling functions. Nature (Lond) 300:752–755
De Greve H, Dhaese P, Seurinck J, Lemmers M, Van Montagu M, Schell J (1983) Nucleotide sequence and transcript map of the Agrobacterium tumefaciens Ti plasmid-encoded octopine synthase gene. J Mol Appl Genet 1:499–511
Denhard DT (1966) A membranefilter technique for the detection of complementary DNA. Biochem Biophys Res Commun 23:641–646
De Vos G, De Beuckeleer M, Van Montagu M, Schell J (1981) Restriction endonuclease mapping of the octopine tumor inducing pTiAch5 of Agrobacterium tumefaciens. Plasmid 6:249–253
Drummond MH, Gordon MP, Nester EW, Chilton M-D (1977) Foreign DNA of bacterial plasmid origin is transcribed in crown gall tumors. Nature (Lond) 269:535–536
Garfinkel DJ, Simpson RB, Ream LW, White FF, Gordon MP, Nester EW (1981) Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell 27:143–153
Gelvin SB, Gordon MP, Nester EW, Aronson AI (1981) Transcription of the Agrobacterium Ti-plasmid in the bacterium and in crown gall tumors. Plasmid 6:17–29
Gurley WB, Kemp JD, Albert MJ, Sutton DW, Callis J (1979) Transcription of Ti plasmid derived sequences in three octopine-type crown gall tumor lines. Proc Natl Acad Sci USA 76:2828–2832
Hernalsteens J-P, Van Vliet F, De Beuckeleer M, Depicker A, Engler G, Lemmers M, Holsters M, Van Montagu M, Schell J (1980) The Agrobacterium tumefaciens Ti plasmid as a host vector system for introducing foreign DNA in plant cells. Nature (Lond) 287:654–656
Joos H, Inze D, Caplan A, Sormann M, Van Montagu M, Schell J (1983) Genetic analysis of T-DNA transcripts in nopaline crown gall. Cell 32:1057–1067
Klapwijk PM, Van Breukelen J, Korevaar K, Ooms G, Schilperoort RA (1980) Transposition of Tn904 encoding Sm resistance into the octopine Ti-plasmid of A. tumefaciens. J Bacteriol 141:129–136
Leemans J, Shaw C, Deblaere R, De Greve H, Hernalsteens J-P, Maes M, Van Montagu M, Schell J (1981) Site-specific mutagenesis of Agrobacterium Ti plasmids and transfer of genes to plant cells. J Mol Appl Genet 1:149–164
Leemans J, Deblaere R, Willmitzer L, De Greve H, Hernalsteens J-P, Van Montagu M, Schell J (1982) Genetic identification of functions of TL-DNA transcripts in octopine crown galls. EMBO J 1:147–152
Lemmers M, De Beuckeleer M, Holsters M, Zambryski P, Depicker A, Hernalsteens J-P, Van Montagu M, Schell J (1980) Internal organization, boundaries and integration of Ti-plasmid DNA in nopaline crown gall tumors. J Mol Biol 144:353–376
Maliga P, Sz Breznovits A, Márton L (1973) Streptomycin-resistant plants from callus culture of haploid tobacco. Nature (Lond) 244:29–30
Márton L, Wullems GJ, Molendijk L, Schilperoort RA (1979) In vitro transformation of cultured cells from Nicotiana tabacum by Agrobacterium tumefaciens. Nature (Lond) 277:129–131
Memelink J, Wullems GJ, Schilperoort RA (1983) Nopaline T-DNA is maintained during regeneration and generative propagation of transformed tobacco plants. Mol Gen Genet 190:516–522
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ooms G, Hooykaas PJJ, Moolenaar G, Schilperoort RA (1981) Crown gall plant tumors of abnormal morphology, induced by Agrobacterium tumefaciens carrying mutated octopine Ti-plasmid; analysis of T-DNA functions. Gene 14:33–50
Otten LABM, Schilperoort RA (1978) A rapid micro scale method for the detection of lysopine and nopaline dehydrogenase activities. Biochim Biophys Acta 527:497–500
Otten L, De Greve H, Hernalsteens J-P, Van Montagu M, Schieder O, Straub J, Schell J (1981) Mendelian transmission of genes introduced into plants by the Ti plasmids of Agrobacterium tumefaciens. Mol Gen Genet 183:209–213
Schell J, Van Montagu M (1977) The Ti plasmid of Agrobacterium tumefaciens, a natural vector for the introduction of NIF genes in plants? In: Hollaender A (ed) Genetic engineering for nitrogen fixation. Plenum Press, New York, pp 159–179
Schell J, Van Montagu M, De Beuckeleer M, De Block M, Depicker A, De Wilde M, Engler G, Genetello C, Hernalsteens J-P, Holsters M, Seurinck J, Silva B, Van Vliet F, Villarroel R (1979) Interactions and DNA transfer between Agrobacterium tumefaciens, the Ti-plasmid and the plant host. Proc R Soc Lond B 204:251–266
Schilperoort RA, Klapwijk PM, Hooykaas PJJ, Koekman BP, Ooms G, Otten LABM, Wurzer-Figurelli EM, Wullems GJ, Rörsch A (1978) A. tumefaciens plasmids as vectors for genetic transformation of plant cells. In: Thorpe TA (ed) Frontiers of plant tissue culture 1978. The International Association for Plant Tissue Culture 1978. University of Calgary, Alberta, Canada, pp 85–94
Schröder J, Schröder G, Huisman H, Schilperoort RA, Schell J (1981) The mRNA for lysopine dehydrogenase in plant tumor cells is complementary to a Ti-plasmid fragment. FEBS Lett 129:166–168
Smith EF, Townsend CO (1907) A plant tumor of bacterial origin. Science 25:671–673
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Thomashow MF, Nutter R, Montoya AL, Gordon MP, Nester EW (1980a) Integration and organization of Ti-plasmid sequences in crown gall tumors. Cell 19:729–739
Thomashow MF, Nutter R, Postle K, Chilton M-D, Blattner FR, Powell A, Gordon MP, Nester EW (1980b) Recombination between higher plant DNA and the Ti plasmid of Agrobacterium tumefaciens. Proc Natl Acad Sci USA 77:6448–6452
Trevelyan W, Proctor D, Harrison J (1950) Detection of sugars on paper chromatograms. Nature 166:444–445
Turgeon R, Wood HN, Braun AC (1976) Studies on the recovery of crown gall tumor cells. Proc Natl Acad Sci USA 73:3562–3564
Van Larebeke N, Engler G, Holsters M, Van den Elsacker S, Zaenen I, Schilperoort RA, Schell J (1974) Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature (Lond) 252:169–170
Watson B, Currier TC, Gordon MP, Chilton M-D, Nester EW (1975) plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol 123:255–264
Willmitzer L, De Beuckeleer M, Lemmers M, Van Montagu M, Schell J (1980) The Ti-plasmid derived T-DNA is present in the nucleus and absent from plastids of plant crown-gall cells. Nature (Lond) 287:359–361
Willmitzer L, Otten L, Simons G, Schmalenbach W, Schröder J, Schröder G, Van Montagu M, De Vos G, Schell J (1981a) Nuclear and polysomal transcripts of T-DNA in octopine crown gall suspension and callus cultures. Mol Gen Genet 182:255–262
Willmitzer L, Schmalenbach W, Schell J (1981b) Transcription of T-DNA in octopine and nopaline crown gall tumors is inhibited by low concentrations of α-amanitin. Nucl Acids Res 9:4801–4812
Willmitzer L, Simons G, Schell J (1982) The TL-DNA in octopine crown gall tumors codes for seven well defined polyadenylated transcripts. EMBO J 1:139–146
Willmitzer L, Dhaese P, Schreier PH, Schmalenbach W, Van Montagu M, Schell J (1983) Size, location and polarity of T-DNA encoded transcripts in nopaline crown gall tumors; common transcripts in octopine and nopaline tumors. Cell 32:1045–1056
Wullems GJ, Molendijk L, Ooms G, Schilperoort RA (1981) Rentention of tumor markers in F1 progeny plants from in vitro induced octopine and nopaline tumor tissues. Cell 24:719–727
Yadav NS, Postle K, Saiki RK, Thomashow MF, Chilton M-D (1980) T-DNA of a crown gall teratoma is covalently joined to host plant DNA. Nature (Lond) 287:458–461
Yang F, Simpson RB (1981) Revertant seedlings from crown gall tumors retain a portion of the bacterial Ti plasmid DNA sequences. Proc Natl Acad Sci USA 78:4151–4155
Zaenen I, Van Larebeke N, Teuchy H, Van Montagu M, Schell J (1974) Supercoiled circular DNA in crown gall inducing Agrobacterium strains. J Mol Biol 86:109–127
Zambryski P, Holsters M, Kruger K, Depicker A, Schell J, Van Montagu M, Goodman HM (1980) Tumor DNA structure in plant cells transformed by A. tumefaciens. Science 209:1385–1391
Author information
Authors and Affiliations
Additional information
Communicated by R. Goldberg
Rights and permissions
About this article
Cite this article
Wöstemeyer, A., Otten, L.A.B.M. & Schell, J.S. Sexual transmission of T-DNA in abnormal tobacco regenerants transformed by octopine and nopaline strains of Agrobacterium tumefaciens . Molec. Gen. Genet. 194, 500–507 (1984). https://doi.org/10.1007/BF00425565
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00425565