Abstract
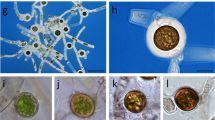
Mannoproteins from cell walls of Saccharomyces cerevisiae synthesized at successive stages of the population growth cycle have been solubilized with Zymolyase and subsequently analyzed. The major change along the population cycle concerned a large size mannoprotein material; the size of the newly-synthesized molecules varied from 120,000–500,000 (mean of about 200,000) at early exponential phase to 250,000–350,000 (mean of about 300,000) at late exponential phase. These differences are due to modifications in the amount of N-glycosidically linked mannose residues, since the size of the peptide moiety was 90,000–100,000 at all growth stages and the level of O-glycosylation changed only slightly. After, incubation of the purified walls with concanavalin A-ferritin and subsequent analysis by electron microscopy, labelling was localized at the external and internal faces of the walls. The middle space of these was labelled after digestion of the glucan network with Zymolyase, which demonstrate the presence of mannoproteins in close contact with the structural glucan molecules throughout the wall.
Similar content being viewed by others
Abbreviations
- BSA:
-
bovine serum albumin
- Con A:
-
concanavalin A
- SDS:
-
sodium dodecyl sulphate
References
Bacon JSD (1981) Nature and disposition of polysaccharides within the cell envelope. In: Arnold WN (ed). Yeast cell envelopes: biochemistry, biophysics and ultrastructure, vol 1. CRC Press, Boca Raton Fla, pp 65–84
Ballou CE (1982) The yeast cell wall and cell surface. In: Strathern JN, Jones EW, Broach JR (eds) The molecular biology of the yeast Saccharomyces, vol 2. Cold Spring Harbor Laboratory, Cold Spring Harbor, pp 335–360
Boucherie H (1985) Protein synthesis during transition and stationary phases under glucose limitation in Saccharomyces cerevisiae. J Bacteriol 161:385–392
Cabib E, Roberts R, Bowers B (1982) Synthesis of the yeast cell wall and its regulation. Annu Rev Biochem 51:763–793
Cassone A, Mattia E, Boldrini L (1978) Agglutination of blastospores of Candida albicans by concanavalin A and its relationship with the distribution of mannan polymers and the ultrastructure of the cell wall. J Gen Microbiol 105:263–273
Deutch CE, Parry JM (1974) Sphaeroplast formation in yeast during the transition from exponential phase to stationary phase. J Gen Microbiol 80:259–266
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method of determination of sugars and related substances. Anal Chem 28:350–356
Elorza MV, Murgui A, Sentandreu R (1985) Dimorphism in Candida albicans: contribution of mannoproteins to the architecture of yeast and mycelial cell walls. J Gen Microbiol 131:2209–2216
Esmon B, Novick P, Schekman R (1981) Compartimentalized assembly of oligosaccharides on exported glycoproteins in yeast. Cell 25:451–460
Frevert J, Ballou CE (1985) Saccharomyces cerevisiae structural cell wall mannoprotein., Biochemistry 24:753–759
Haselbeck A, Tanner W (1983) O-glycosylation in Saccharomyces cerevisiae is initiated at the endoplastic reticulum. FEBS Lett 158:335–338
Herrero E, Valentín E, Sentandreu R (1985) Effect of α-factor on individual wall mannoproteins from Saccharomyces cerevisiae a cells. FEMS Microbiol Lett 27:293–297
Horisberger M, Volanthen M (1977) Location of mannan and chitin on thin sections of budding yeasts with gold markers. Arch Microbiol 115:1–7
Horisberger M, Rouvet-Vauthey M, Richli U, Farr DR (1985) Cell wall architecture of the halophilic yeast Saccharomyces rouxii. An immunocytochemical study. Eur J Cell Biol 37:70–77
Kitamura K, Kaneko T, Yamamoto Y (1974) Lysis of viable yeast cells by enzymes of Arthrobacter luteus. II. Purification and properties of an enzyme, zymolyase, which lyses viable yeast cells. J Gen Appl Microbiol 20:323–344
Murgui A, Elorza MV, Sentandreu R (1986) Tunicamycin and papulacandin B inhibit incorporation of specific mannoproteins into the walls of Candida albicans regenerating protoplasts. Biochim Biophys Acta 884:550–558
Nakajima T, Ballou CE (1974) Structure of the linkage region between the polysaccharide and protein parts of Saccharomyces cerevisiae mannan. J Biol Chem 249:7685–7694
Northcote DH, Horne RW (1952) The chemical composition and structure of the yeast cell wall. Biochem J 51:232–236
Novick P, Schekman R (1983) Export of major cell surface proteins is blocked in yeast secretory mutants. J Cell Biol 96:541–547
Pastor FIJ, Valentín E, Herrero E, Sentandreu R (1984) Structure of the Saccharomyces cerevisiae cell wall: mannoproteins released by zymolyase and their contribution to wall architecture. Biochim Biophys Acta 802:292–300
Sentandreu R, Northcote, DH (1969) The characterization of oligosaccharides attached from the cell wall of yeast. Carbohydr Res 10:584–585
Sentandreu R, Herrero E, Martínez JP, Larriba G (1984) Biogenesis of the yeast cell wall, Subcell Biochem 10:193–235
Sharma CB, Babczinski P, Lehle L, Tanner W (1974) The role of dolichol monophosphate in glycoprotein biosynthesis in Saccharomyces cerevisiae. Eur J Biochem 46:35–41
Shibata N, Mizugami K, Takano K, Suzuki S (1983) Isolation of mannanprotein complexes from viable cells of Saccharomyces cerevisiae X-2180-1A wild type and Saccharomyces cerevisiae X-2180-1A-5 mutant strains by the action of Zymolyase-60,000. J Bacteriol 156:552–558
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastr Res 26:31–43
Trimble RB, Maley F (1977) Subunit structure of external invertase from Saccharomyces cerevisiae. J Biol Chem 252:4409–4412
Tronchin G, Poulain D, Herbaut J, Biguet J (1981) Cytochemical and ultrastructural studies of Candida albicans. II. Evidence for a cell wall coat using concanavalin A. Ultrastr Res 75: 50–59
Valentín E, Herrero E, Sentandreu R (1986) Incorporation of mannoproteins into the walls of aculeacin A-treated yeast cells. Arch Microbiol 146:214–220
Villanueva JR (1966) Protoplasts in fungi. In: Ainsworth GC, Sussman AE (eds) The fungi, vol 2. Academic Press, London New York, pp 3–62
Zlotnik H, Fernández MP, Bowers B, Cabib E (1984) Saccharomyces cerevisiae mannoproteins from an external cell wall layer that determines wall porosity. J Bacteriol 159:1018–1026
Zueco J, Mormeneo S, Sentandreu R (1986) Temporal aspects of the O-glycosylation of Saccharomyces cerevisiae mannoproteins. Biochim Biophys Acta 884:93–100
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Valentín, E., Herrero, E., Rico, H. et al. Cell wall mannoproteins during the population growth phases in Saccharomyces cerevisiae . Arch. Microbiol. 148, 88–94 (1987). https://doi.org/10.1007/BF00425354
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00425354