Summary
-
1.
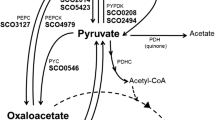
Spirochaeta aurantia fermented glucose-1-14C primarily to ethanol, acetate, CO2, and H2. Most of the 14C-label was recovered from carbon 2 of ethanol and acetate, whereas essentially no radioactivity was present in CO2. Phosphofructokinase, fructosediphosphate aldolase, triosephosphate isomerase, and glyceraldehydephosphate dehydrogenase activities were detected in cell extracts. These data indicate that S. aurantia ferments glucose to pyruvate via the Embden-Meyerhof pathway.
-
2.
Whole cells and cell extracts exhibited a coenzyme A-dependent CO2-pyruvate exchange. No formate-pyruvate exchange was detected, nor was formate involved in CO2 or H2 production. Acetyl phosphate formation from pyruvate by cell extracts was markedly stimulated by the presence of CoA in reaction mixtures. It was concluded that the organism utilizes a clostridial-type phosphoroclastic system to form acetyl-coenzyme A, CO2, and H2 from pyruvate. Acetyl CoA is metabolized to acetate via phosphotransacetylase and acetate kinase, or converted to ethanol by a double reduction involving aldehyde and alcohol dehydrogenase activities.
-
3.
A rubredoxin, purified from cell extracts of S. aurantia, exhibited absorption maxima at 275, 376, and 490 nm (oxidized), and 275, 312, and 336 nm (NaBH4-reduced).
Similar content being viewed by others
References
Avison, A. W. D.: The synthesis of acyl phosphates in aqueous solution. J. chem. Soc. 1955, 732–738 (1955).
Breznak, J. A., Canale-Parola, E.: Spirochaeta aurantia, a pigmented, facultatively anaerobic spirochete. J. Bact. 97, 386–395 (1969).
—— Metabolism of Spirochaeta aurantia. II. Aerobic oxidation of carbohydrates. Arch. Mikrobiol. 83, 278–292 (1972).
Chase, G. D.: Principles of radioisotope methodology. Minneapolis, Minn.: Burgess Publishing Co. 1959.
Dawes, E. A., Foster, S. M.: The formation of ethanol in Escherichia coli. Biochim. biophys. Acta (Amst.) 22, 253–265 (1956).
Dolin, M. I., Gunsalus, I. C.: Pyruvic acid metabolism. II. An acetoin-forming enzyme system in Streptococcus faecalis. J. Bact. 62, 199–214 (1951).
Hespell, R. B.: Physiology and energy metabolism in Spirochaeta litoralis, Spirochaeta stenostrepta, and Treponema denticola, Dissertation, University of Massachusetts, 1970.
— Canale-Parola, E.: Carbohydrate metabolism in Spirochaeta stenostrepta. J. Bact. 103, 216–226 (1970a).
—— Spirochaeta litoralis sp.n., a strictly anaerobic marine spirochete. Arch. Mikrobiol. 74, 1–18 (1970b).
—— Amino acid and glucose fermentation by Treponema denticola. Arch. Mikrobiol. 78, 234–251 (1971).
— Joseph, R., Mortlock, R. P.: Requirement for coenzyme A in the phosphoroclastic reaction of anaerobic bacteria. J. Bact. 100, 1328–1334 (1969).
Horecker, B. L., Kornberg, A.: The extinction coefficients of the reduced band of pyridine nucleotides. J. biol. Chem. 175, 385–390 (1948).
Koepsell, H. J., Johnson, M. J.: Dissimilation of pyruvic acid by cell-free preparations of Clostridium butylicum. J. biol. Chem. 145, 379–386 (1942).
Kupfer, D. G., Canale-Parola, E.: Pyruvate metabolism in Sarcina maxima. J. Bact. 94, 984–990 (1967).
Lipmann, F., Tuttle, L. C.: A specific micromethod for the determination of acyl phosphates. J. biol. Chem. 159, 21–28 (1945).
Lovenberg, W., Sobel, B. E.: Rubredoxin: a new electron transfer protein from Clostridium pasteurianum. Proc. nat. Acad. Sci. (Wash.) 54, 193–199 (1965).
McCormick, N. G., Ordal, E. J., Whiteley, H. R.: Degradation of pyruvate by Micrococcus lactilyticus. I. General properties of the formate-exchange reaction. J. Bact. 83, 887–898 (1962).
Mortlock, R. P., Valentine, R. C., Wolfe, R. S.: Carbon dioxide activation in the pyruvate clastic system of Clostridium butyricum. J. biol. Chem. 234, 1653–1656 (1959).
Neish, A. C.: Analytical methods for bacterial fermentations, Nat. Res. Council of Canada, Report No. 46-8-3 (2nd revision), Saskatoon 1952.
Newman, D. J., Postgate, J. R.: Rubredoxin from a nitrogen-fixing variety of Desulfovibrio desulfuricans. Europ. J. Biochem. 7, 45–50 (1968).
Peck, H. D., Jr., Gest, H.: Formic dehydrogenase and the hydrogenlyase enzyme complex in coli-aerogenes bacteria. J. Bact. 73, 706–721 (1957).
Pelnoy, R. A., Whiteley, H. R.: Regulatory properties of acetokinase from Veillonella alcalescens. J. Bact. 105, 259–267 (1971).
Peterson, J. A., Coon, M. J.: Enzymatic ω-oxidation. III. Purification and properties of rubredoxin, a component of the ω-hydroxylation system of Pseudomonas oleovorans. J. biol. Chem. 243, 329–334 (1968).
— Kusunose, M., Kusunose, E., Coon, M. J.: Enzymatic ω-oxidation. II. Function of rubredoxin as the electron carrier in ω-hydroxylation. J. biol. Chem. 242, 4334–4340 (1967).
Postgate, J. R.: Cytochrome c3 and desulphoviridin: pigments of the anaerobe Desulphovibrio desulphuricans. J. gen. Microbiol. 14, 545–572 (1956).
Rose, I. A., Grunberg-Manago, M., Korey, S. R., Ochoa, S.: Enzymatic phosphorylation of acetate. J. biol. Chem. 211, 737–756 (1954).
Shanmugam, K. T., Arnon, D. I.: Isolation and characterization of two types of ferredoxin from a photosynthetic bacterium. Fed. Proc. 30, 1136 (1971).
Stadtman, E. R., Novelli, G. D., Lipmann, F.: Coenzyme A function in and acetyl transfer by the phosphotransacetylase system. J. biol. Chem. 191, 365–376 (1951).
Suh, B., Akagi, J. M.: Pyruvate-carbon dioxide exchange reaction of Desulfovirio desulfuricans. J. Bact. 91, 2281–2285 (1966).
Umbreit, W. W., Burris, R. H., Stauffer, J. F.: Manometric techniques. Minneapolis, Minn.: Burgess Publishing Co. 1964.
Vinzent, R.: Isolement et culture de spirilles et de spirochètes des eaux. C. R. Soc. biol. (Paris) 95, 1472–1474 (1926).
Warburg, O., Christian, W.: Isolierung und Kristallisation des Gärungsferments Enolase. Biochem. Z. 310, 384–421 (1942).
Whiteley, H. R., McCormick, N. G.: Degradation of pyruvate by Micrococcus lactilyticus. III. Properties and cofactor requirements of the carbon dioxide-exchange reaction. J. Bact. 85, 382–393 (1963).
Wolfe, R. S., O'Kane, D. J.: Cofactors of the phosphoroclastic reaction of Clostridium butyricum. J. biol. Chem. 205, 755–765 (1953).
—— Cofactors of the carbon dioxide exchange reaction of Clostridium butyricum. J. biol. Chem. 215, 637–643 (1955).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Breznak, J.A., Canale-Parola, E. Metabolism of Spirochaeta aurantia . Archiv. Mikrobiol. 83, 261–277 (1972). https://doi.org/10.1007/BF00425239
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00425239