Abstract
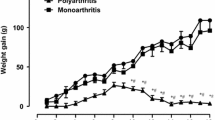
A foot-pad oedema model was used to investigate some early peripheral blood changes associated with the development of an acute inflammatory response. The inflammatory model used was an example of a cell-mediated hypersensitivity reaction. Male rats were inoculated in the scruff with Freund's Complete Adjuvant (FCA) on day 0 and then challenged 6 days later with FCA in one hind paw. An acute inflammatory reaction was initiated over the following 72 hours. Within 8 hours of induction a significant increase in neutrophils was detected in the peripheral blood reaching peak levels at 24 hours. The peak level of monocytes was not seen until 48 hours after challenge. Lymphocyte numbers, haemoglobin and haematocrit values all remained within the normal range. Plasma iron levels fell by approximately 85% within 24 hours whereas plasma α-1-acid glycoprotein and the copper-containing protein caeruloplasmin were found to increase steadily throughout the development of the inflammatory response. These findings are discussed in relation to previously published work using the same model. An important inter-relationship between phagocytic cells, iron and free radical production at the site of the acute inflammatory reaction is suggested.
Similar content being viewed by others
References
Babior BM (1978) Oxygen dependent microbial killing by phagocytes. N Engl J Med 298: 659–668
Billingham MEJ, Gordon AH (1976) The role of the acute phase reaction in inflammation. Agents Actions 6: 195–199
Birgegard G, Caro J (1984) Increased ferritin synthesis and iron uptake in inflammatory mouse macrophages. Scand J Haematol 33: 43–48
Bullock GP, Christmas S, Jasani MK, Peters RF, Tweed MF, Williamson IHM (1983) Lymphocytes, inflammation and fibrin studies in an experimental foot-oedema model of the Koch phenomenon. Br J Pharmacol 79: 240P
Ceriotti F, Ceriotti G (1980) Improved direct specific determination of serum iron and total iron-binding capacity. Clin Chem 26: 327–331
Dacie JV, Lewis SM (1984) Practical haematology, 6th edn. Churchill Livingstone London
Dowling EJ (1985) The role of lipid peroxidation in inflammation, PhD thesis, University of Surrey
Dowling EJ, Symons AM, Parke DV (1986) Free radical production at the site of an acute inflammatory reaction as measured by chemiluminescence. Agents Actions 19: 203–207
Frieden E (1986). Perspectives on copper biochemistry. Clin Physiol Biochem 4: 11–19
Frieden E, Hsieh HS (1976) Caeruloplasmin: the copper transport protein with essential oxidase activity. Adv Enzymol 44: 187–236
Gauldie J, Lamontagne L, Stadnyk A (1985) Acute phase response in infectious disease. Surv Synth Path Res 4: 126–151
Glenn EM, Gray J, Kooyers W (1965) Chemical changes in adjuvantinduced polyarthritis of rats. Am J Vet Res 26: 1195–1203
Goldstein IM, Kaplan HB, Edelson HS, Weissman G (1982) Caeruloplasmin: an acute phase reactant that scavenges oxygen-derived free radicals. Ann NY Acad Sci 389: 368–379
Gutteridge JMC, Stocks J (1981) Caeruloplasmin: physiological and pathological perspectives. CRC Crit Rev Clin Lab Sci 14: 257–329
Haber F, Weiss J (1934) The catalytic decomposition of H2O2 by iron salts. Proc R Soc Series A 147: 332–351
Halliwell B (1985) Metal ions, free radical reactions and inflammation: an assessment of current status. In: Venge P, Lindbom A (eds), Inflammation, basic mechanisms, tissue injuring principles and clinical models. Almqvist and Wiksell Int. Sweden
Hawkey CM (1975) Comparative mammalian haematology. Heinemann Medical Books, London
Konijn AM, Hershko C (1977) Ferritin synthesis in inflammation. 1. Pathogenesis of impaired iron release. Br J Haematol 37: 7–16
Laine E, Couderc R, Roch-Arveiller M, Vasson MP, Giroud JP, Raichvarg D (1990) Modulation of human polymorphonuclear neutrophil functions by α1-acid glycoprotein. Inflammation 14: 1–9
Letendre ED (1985) The importance of iron in the pathogenesis of infection and neoplasia. Trends in Biochem Sci 10: 166–168
Lewis DA (1984) Endogenous anti-inflammatory factors. Biochem Pharmacol 33: 1705–1714
Lukens JN, Cartwright GE, Wintrobe MM (1967) Anaemia of adjuvant-induced inflammation in rats. Proc Soc Exp Biol Med 126: 346–349
Mikolajew M, Kuratowska Z, Kossakowska M, Plachecka M, Kopec M (1969) Haematological changes in adjuvant disease in the rat. 1. Peripheral blood and bone marrow after repeated injections of Freund's adjuvant. Ann Rheum Dis 28: 35–40
Mori L, Bekkering A, Traini J, Vanderlinden L (1981) Ferrozine iron and total iron-binding capacity method adapted to the ABA-100 Biochromatic analyser. Clin Chem 27: 1441–1444
Osaki S, Johnson DA, Frieden E (1966) The possible significance of the ferrous oxidase activity of caeruloplasmin in normal human serum. J Biol Chem 241: 2746–2751
Pearson CM (1956) Development of arthritis, periarthritis and periostitis in rats given adjuvant. Proc Soc Exp Biol (NY) 91: 95–101
Piercy DWT (1979) Acute phase responses to experimental salmoneliosis in calves and colibacillosis in chickens: serum iron and caeruloplasmin. J Comp Pathol 89: 309–319
Piliero SJ, Graeme ML, Sigg EB, Chinea G, Columbo C (1966) Action of anti-inflammatory agents upon blood and histopathological changes induced by periarthritis in rats. Life Sci 5: 1057–1069
Schalm OW, Jain NC, Carroll EJ (1975) Veterinary haematology 3rd edn. Lea and Febiger, Philadelphia
Schlosnagle DC, Hutton PS, Conn RB (1982) Ferrozine assay of serum iron and total iron-binding capacity adapted to the Cobas Bio centrifugal analyser. Clin Chem 28: 1730–1732
Sunderman FW, Nomoto S (1970) Measurement of human serum caeruloplasmin by its p-phenylenediamine oxidase activity. Clin Chem 16: 903–909
Venge P, Lindbom A (1985) Inflammation, basic mechanisms, tissue injuring principles and clinical models, Almqvist and Wiksell International, Sweden
Wedmore CV, Williams TJ (1981) Control of vascular permeability by polymorphonuclear leucocytes in inflammation. Nature (Lond) 289:646–650
Weinberg ED (1984) Iron withholding: a defense against infection and neoplasia. Physiol Rev 64: 65–102
Whicher JT (1984) Functions of acute phase proteins in the inflammatory response. In: Arnauld P, Bienvenu J, Laurent P (eds) Marker proteins in inflammation volume 2. Walter de Gruyter, Berlin, New York
Willoughby DA (1987) Inflammation-mediators and mechanisms. Br Med Bull 43: 247–477
Wintrobe MM, Greenberg GR, Humphreys S, Ashenbrucker H, Worth W, Kramer R (1947) The anaemia of infection. III. The uptake of radioactive iron in iron-deficient and in pyridoxinedeficient pigs before and after acute inflammation. J Clin Invest 26: 103–113
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Howarth, J.A., Hall, D.E., Dowling, E.J. et al. Haematological changes and free-radical involvement in an experimental foot-pad oedema model. Comparative Haematology International 1, 42–48 (1991). https://doi.org/10.1007/BF00422692
Issue Date:
DOI: https://doi.org/10.1007/BF00422692