Summary
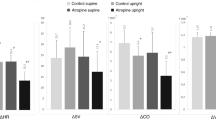
The effects of atropine upon changes in the circulating levels of growth hormone (GH), cortisol, lactate, glucose, and free fatty acids (FFA) were studied during exercise using both constant and progressively increasing work loads. At low work loads, atropine had no effect upon the changes in either cortisol or lactate levels, but the normal exercise-induced rise in GH was abolished or markedly reduced. At higher work loads, especially when prolonged, the usual rises in cortisol and lactate were enhanced by atropine, but the rise in GH was diminished and delayed. In no circumstances were the changes in FFA or glucose significantly affected by atropine.
We regard the effect of atropine upon changes in cortisol and lactate responses as secondary to its cardiovascular effect, but suggest that the inhibition of GH release may be evidence of a cholinergic mechanism in the control of GH release during exercise.
Similar content being viewed by others
References
Bruni JF, Meites J (1978) Effects of cholinergic drugs on growth hormone release. Life Sci 23:1351–1358
Cashmore GC, Few JD, Fowles CH (1976) Reliability of plasma cortisol determination by a simple competitive protein binding method. Ann Clin Biochem 13:371–378
Daughaday WH, Kipnis DM (1966) The growth promoting and anti-insulin actions of somatotropin. Rec Prog Horm Res 22:49–99
Davies CTM, Brotherhood JR, Few JD, ZeidiFard E (1976) Effects of Β-blockade and atropinisation on plasma catecholamine concentration during exercise. Eur J Appl Physiol 36:49–56
Davies CTM, Few JD (1973) The effect of exercise on adrenocortical function. J Appl Physiol 35:887–891
Davies CTM, Few JD (1976) The effect of hypoxia on the adrenocortical response to exercise in man. J Endocrinol 71:157–158
Duncombe WG (1964) The colorimetric microdetermination of non-esterified fatty acids in plasma. Clin Chim Acta 9:122–125
Few JD, Cashmore GC (1971) The determination of plasma cortisol concentration by competitive protein binding. Ann Clin Biochem 8:205–209
Ganong WF (1974) The role of catecholamines and acetylcholine in the regulation of endocrine function. Life Sci 15:1401–1414
Hansen AP (1971) The effect of adrenergic receptor blockade on the exercise-induced serum growth hormone rise in normals and juvenile diabetics. J Clin Endocrinol 33:807–812
Hayden PW, Larson SM, Lakshminarayanan S (1979) Atropine clearance from human plasma. J Nucl Med 20:366–367
Hunter WM, Fonseka CC, Passmore R (1965) The role of GH in the mobilisation of fuel for muscular exercise. Q J Exp Physiol 50:406–416
Hunter WM, Fonseka CC, Passmore R (1965) Growth hormone, important role in muscular exercise in adults. Science 150:1051–1053
McCullough R (1968) Semi-automated method for the differential determination of plasma catecholamines. J Clin Pathol 21:759–763
Mendelson WB, Sitaram N, Wyatt RJ, Gillin JP (1978) Methscopolamine inhibition of sleep-related Growth Hormone Secretion. J Clin Invest 61:1683–1689
Métivier G (1975) Effects of long lasting physical exercise and training on hormonal regulation. In: Howald H, Poortmans JR (eds) Metabolic adaptation to prolonged physical exercise. BirkhÄuser, Basel, pp 276–292
Pruett EDR (1970) Glucose and insulin during prolonged work stress in men living on different diets. J Appl Physiol 28:199–208
Raben MS, Hollenberg CH (1959) Effect of growth hormone on plasma fatty acids. J Clin Invest 38:484–488
Smythe GA, Lazarus L (1974) Suppression of human growth hormone secretion by melatonin and cyprohentadine. J Clin Invest 54:116–121
Sutton J, Lazarus L (1974) Effect of adrenergic blocking agents on growth hormone responses to physical exercise. Horm Metab Res 6:428–429
Young PW, Bicknell RJ, Schofield JG (1979) Acetylcholine stimulates growth hormone secretion, phosphatidyl inositol labelling, 45Ca2+ Efflux and cyclic GHP accumulation in bovine anterior pituitary glands. J Endocrinol 80:203–213
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Few, J.D., Davies, C.T.M. The inhibiting effect of atropine on growth hormone release during exercise. Europ. J. Appl. Physiol. 43, 221–228 (1980). https://doi.org/10.1007/BF00421835
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00421835