Summary
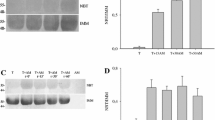
Accumulation of tubulin as compared with the accumulation of total cellular protein in human NHIK 3025 cells treated with the sulfone 2-(2-thenyl)sulfonyl-5-bromopyrimidine (NY 4137) and the sulfoxide 2-(2thenyl)sulfinyl-5-bromopyrimidine (NY 4138), two mitotic inhibitors, were investigated by two-parametric flow cytometry. Following a 4 h treatment with NY 4137 tubulin accumulation is inhibited while total protein continues to accumulate. After treatment for 4h with NY 4138 the accumulation of total protein is approximately constant, while the accumulation of tubulin is reduced although not to the same degree as that found for NY 4137-treated cells. In addition, the percentage tubulin SH-groups (6.89 ± 0.14) remaining after treatment of purified rat brain tubulin with NY 4137 or NY 4138 was determined. Treatment with 0.0125 mM NY 4137 reduced the number of tubulin SH-groups detectable with dithiobis benzoate or from 6.89 ± 0.14 before treatment to about 4 after treatment. However, practically all SH-groups of tubulin remain detectable following treatment with the same concentration of NY 4138. From the results described in this report we infer that NY 4137 binds to tubulin SH-groups and that inhibition of tubulin accumulation follows as a secondary effect.
Similar content being viewed by others
References
Pettersen EO, Nome O, Dornish JM, Oftebro R: Mitotic arrest and interphase inhibition induced by the pyrimidine sulfoxide NY 4138. Invest New Drugs 3: 245–253, 1985
Nygaard MEH, Dornish JM, Oftebro R, Undheim K: Effects of the new mitotic inhibitor pyrimidinesulfone NY 4137 on human cells in vitro and on colchicine binding to tubulin. Invest New Drugs 4: 221–229, 1986
Nygaard MEH, Pettersen EO, Dornish JM, Undheim K, Oftebro R: Inactivation of human cells cultivated in vitro by the new mitotic inhibitors NY 4137 and NY 4138. Invest New Drugs 5: 259–266, 1987
Nygaard MEH, Dornish JM, Pettersen EO, Oftebro R: Sulfhydryl-related reduction of the mitotic inhibition and cell inactivation induced by pyrimidine analogs. Anti-Cancer Drug Design 3: 15–24, 1988
Bryan I, Wilson L: Are cytoplasmic microtubules heteropolymers. Proc Natl Acad Sci USA 68: 1762–1766, 1971
Bryan I. Vinblastine and microtubules characterization of two protein subunits from the isolated crystals. J Mol Biol 66:157–168, 1972
Lee JC, Frigon RP, Timascheff SN: The chemical characterization of microtubule protein subunits. J Biol Chem 218:7253–7262, 1973
Luduena RF, Woodward DO: Isolation and Partial Characterization of α- and β-Tubulin from Outer Doublets of Sea-Urchin Sperm and Microtubules of Chick-Embryo Brian. Proc natn Acad Sci USA 70: 3594–3598, 1973
Kuriyama R, Sakai H: Role of tubulin-SH groups in polymerization to microtubules. J Biochem 76: 651–654, 1974
Mellon MG, Rebhun L: Studies on the accessible sulfhydryls of polymerizable tubulin. In: Cell motility. Goldman R, Pollard T, Rosenbaum I (eds), p 1149. Cold Spring Harbor Laboratory, N.Y., 1976a
Oliver JM, Albertini DF, Berlin RD: Effects of glutathione-oxidizing agents on microtubule assembly and microtubule-dependent surface properties of human neutrophils. J Cell Biol 71: 921–932, 1976
Nishida E, Kobayashi T: Relationship between tubulin SH groups and bound guanine nucleotides. J Biochem (Tokyo) 81:343–347, 1977
Burchill BR, Oliver JM, Pearson CB, Leinbach ED, Berlin RD: Microtubule dynamics and glutathione metabolism in phagocytizing human polymorphonuclear leukocytes. J Cell Biol 76: 439–447, 1978
Taylor EW: The mechanism of colchicine inhibition of mitosis. I. Kinetics of inhibition and the binding of 3H-colchicine. J Cell Biol 25: 145–160, 1965
Debrabander MJ, van de Veire RML, Aerts FEM, Borgers M, Janssen PAJ: The effects of methyl [5-(2-thienylcarbonyl)-methyl-1H-benzinicozol-2-yl] carbamate (R 17 934: NSC 238159), a new synthetic antitumoral drug interfering with microtubules, on mammalian cells cultured in vitro. Cancer Res 36: 905–916, 1976
Bryan I: Vinblastine and microtubules. I. Induction and isolation of crystals from sea urchin oocytes. Biochemistry 11:2611–2616, 1971
Schiff PB, Fant I., Horwitz SB: Promotion of microtubule assembly in vitro by taxol. Nature (Lond.) 277: 665–667, 1979.
Schiff PB, Horwitz SB: Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA 77: 1561–1565, 1980
Nordbye K, Oftebro R: Establishment of four new cell strains from human uterine cervix. I. Exp Cell Res 58: 458, 1969
Oftebro R, Nordbye K: Establishment of four new cell strains from human uterine cervix. II. Exp Cell Res 58: 459–460, 1969
Puck TT, Cieciura SJ, Fisher HW: Clonal growth in vitro of human cells with fibroblastic morphology. J Exp Med 106: 145–165, 1957
Pettersen EO, Bakke O, Lindmo T, Oftebro R: Cell cycle characteristics of synchronized and asynchronous population of human cells and effect of cooling and selected mitotic cells. Cell Tissue Kinet 10: 511–522, 1977
Eipper BA: Rat brain microtubule protein: purification and determination of covalently bound phosphate and carbohydrate. Proc Natl Acad Sci USA 69: 2283–2287, 1972
Ellman GL: Tissue sulfhydryl groups. Arch Biochem Biophys 82: 70–77, 1959
Lindmo T, Steen HB: Flow cytometric measurement of the polarization of fluorescence from intracellular fluorescein in mammalian cells. Biophys J 18: 173–187, 1977
Fosså SD, Thorud E, Shoaib MC, Pettersen EO, Høie J, Scott Knudsen O: DNA flow cytometry in primary breast carcinoma. Acta path microbiol scand sect 4, 92: 475–489, 1984
Benneche T, Gacek MJ, Undheim K: Pyrmidine-2-sulphides and their s-oxides for use in medicine and methods of use therefore, pharmaceutical compositions containing them, processes for their preparation and such compounds when novel per se. Eur Pat appl 0 033: 195, 1981
Dornish JM, Randen I, Juul N, Pettersen EO: Is accumulation of actin restricted to G1? Biochem Soc Trans 15: 856–866, 1987
Juul NO, Randen I, Dornish JM, Oftebro R, Pettersen EO: Program for rescaling and normalization of 2-parametric DNA versus protein histograms making use of an internal standard for both parameters. Cytometry supply 2 83: 552A, 1988
Mazia D: SH compounds in mitosis. I. The action of mercaptoethanol on the eggs of the sand dollar dendraster excentricus. Exp Cell Res 14: 486–494, 1958
Kuriyama R: In vitro polymerization of marine egg tubulin into microtubules. J Biochem 81: 1115–1125, 1976
Kuriyama R: In vitro polymerization of flagellar and cilliary outer fiber tubulin into microtubules. J Biochem 80: 153–165, 1977
Ben-Ze'ev AS, Farmer R, Penman S: Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell 17:319–325, 1979
Cleveland DW: The tubulins: From DNA to RNA to protein and back again. Cell 34: 330–332, 1983
Cleveland DW, Havercroft JC: Is apparent autoregulatory control of tubulin synthesis non-transcriptionally regulated? J Cell Biol 97: 919–924, 1983
Cleveland DW, Pittenger MF, Feramisco JR: Elevation of tubulin levels by microinjection suppresses new tubulin synthesis. Nature 305: 738–740, 1983
Caron J, Jones AL, Rall LB, Kirschner MW: Autoregulation of tubulin synthesis in enucleated cells. Nature 317: 648–650, 1985
Pittenger MF, Cleveland DW: Retention of autoregulatory control of tubulin synthesis in cytoplasts: demonstration of a cytoplasmic mechanism that regulates the level of tubulin expression. J Cell Biol 101: 1941–1952, 1985
Yen TJ, David AG, Pachter JS, Cleveland DW: Autoregulated changes in stability of polyribosome-bound β-tubulin mRNAs are specified by the first 13 translated nucleotides. Mol Cell Biol 8: 1224–1235, 1988
Rønning ØW, Seglen PO: The relation between protein accumulation and cell cycle traverse of human NHIK 3025 cells in unbalanced growth. J Cell Phys 112: 19–26, 1982
Rønning Ø, Lindmo T: Progress through G1 and S in relation to net protein accumulation in human NHIK 3025 cells. Exp Cell Res 144: 1–8, 1983
Luduena R, Roach MC: Interaction of tubulin with drugs and alkylating agents. 2. Effects of colchicine, podophyllotoxin and vinblastine on the alkylation of tubulin. Biochemistry 20: 4444–4450, 1975
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holm Juul, M.E., Dornish, J.M., Juul, N.O. et al. Effect of two pyrimidine analogs on accumulation of tubulin in NHIK 3025 cells. Mol Cell Biochem 96, 117–126 (1990). https://doi.org/10.1007/BF00420903
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00420903