Abstract
Enzyme production in a cell recycle fermentation system was studied by computer simulations, using a mathematical model of α-amylase production by Bacillus amyloliquefaciens. The model was modified so as to enable simulation of enzyme production by hypothetical organisms having different production kinetics at different fermentation conditions important for growth and production. The simulations were designed as a two-level factorial assay, the factor studied being fermentation with or without cell recycling, repression of product synthesis by glucose, kinetic production constants, product degradation by a protease, mode of fermentation, and starch versus glucose as the substrate carbon source.
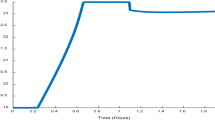
The main factor of importance for ensuring high enzyme production was cell recycling. Product formation kinetics related to the stationary growth phase combined with continuous fermentation with cell recycling also had a positive impact. The effect was greatest when two or more of these three factors were present in combinations, none of them alone guaranteeing a good result. Product degradation by a protease decreased the amount of product obtained; however, when combined with cell recycling, the protease effect was overshadowed by the increased production. Simulation of this type should prove a useful tool for analyzing troublesome fermentations and for identifying production organisms for further study in integrated fermentation systems.
Similar content being viewed by others
Abbreviations
- a :
-
proportionality constant relating the specific growth rate to the logarithm of G (h)
- a 1 :
-
reaction order with respect to starch concentration
- a 2 :
-
reaction order with respect to glucose concentration
- c :
-
starch concentration (g/l)
- c 0 :
-
starch concentration in the feed (g/l)
- D :
-
dilution rate (h−1)
- e :
-
intrinsic intracellular amylase concentration (g product/g cell mass)
- E :
-
extracellular amylase concentration (g/l)
- F :
-
volumetric flow rate (l/h)
- G :
-
average number of genome equivalents of DNA/cell
- K 1 :
-
intracellular repression constant
- K 2 :
-
intracellular repression constant
- K s :
-
Monod saturation constant (g/l)
- k 3 :
-
product excretion rate constant (h−1)
- k I :
-
translation constant (g product/g mRNA/h)
- k d :
-
first order decay constant (h−1)
- k dw :
-
first order decay constant (h−1)
- k gl :
-
rate constant for glucose production (g/l/h)
- k m, dgr :
-
saturation constant for product degradation (g/l)
- k st :
-
rate constant for starch hydrolysis (g/l/h)
- k t1 :
-
proportionality constant for amylase production (g mRNA/g substrate)
- k t2 :
-
proportionality constant for amylase production (g mRNA *h/g substrate)
- k w :
-
protease excretion rate constant (h−1)
- k wt1 :
-
proportionality constant for protease production (g mRNA/g substrate)
- k wt2 :
-
proportionality constant for protease production (g mRNA *h/g substrate)
- k wI :
-
translation constant (g protease/g mRNA/h)
- m :
-
maintenance coefficient (g substrate/g cell mass/h)
- n :
-
number of binding sites for the co-repressor on the cytoplasmic repressor
- Q :
-
repression function, K1/K2 less than or equal to 1.0
- Q w :
-
repression function, K1/K2 less than or equal to 1.0
- r :
-
intrinsic amylase mRNA concentration (g mRNA/g cell mass)
- r m :
-
intrinsic protease mRNA concentration (g mRNA/g cell mass)
- R ex :
-
retention by the filter of the compounds x=: C starch, E amylase, or S glucose
- R t :
-
amylase transport rate (g product/g cell mass/h)
- R wt :
-
protease transport rate (g protease/g cell mass/h)
- R s :
-
rate of glucose production (g/l/h)
- R c :
-
rate of starch hydrolysis (g/l/h)
- S 0 :
-
feed concentration of free reducing sugar (g/l)
- s :
-
extracellular concentration of reducing sugar (g/l)
- t :
-
time (h)
- V :
-
volume (1)
- w :
-
intracellular protease concentration (g/l)
- W :
-
extracellular protease concentration (g/l)
- X :
-
cell mass concentration (dry weight) (g/l)
- Y :
-
yield coefficient (g cell mass/g substrate)
- σ :
-
substrate uptake (g substrate/g cell mass/h)
- μ :
-
specific growth rate of cell mass (h−1)
- μ d :
-
specific death rate of cells (h−1)
- μ m :
-
maximum specific growth rate of cell mass (h−1)
- μ m,dgr :
-
maximum specific rate of amylase degradation (h−1)
References
Groot, W.J.; Sikkenk, C.M.; Waldram, R.H.; van der Lans, R.G.J.J.M.; Luyben, K.C.A.M.: Kinetics of ethanol production by baker's yeast in an integrated process of fermentation and microfiltration. Bioprocess Eng. 8 (1992) 39–47
Nipkow, A.; Sonnleiter, B.; Fichter, A.: Performance of a filter loop reactor using Zymomonas mobilis and validation of the ethanol production model-II. J. Biotechnol. 4 (1986) 46–61
Lee, C.W.; Chang, H.N.: Kinetics of ethanol fermentations in membrane cell recycle fermentors. Biotechnol. Bioeng. 29 (1987) 1105–1112
Reed, W.M.; Bogdan, M.E.: Application of cell recycle to continuous fermentative acetic acid production. Biotechnol. Bioeng. Symp. 8 (1985) 641–647
Enzminger, J.D.; Asenjo, J.A.: Use of cell recycle in the aerobic fermentative production of citric acid by yeast. Biotechnol. Lett. 8 (1986) 7–12
Denis, S.; Boyaval, P.: Microbial enzyme production in a membrane bioreactor. Appl. Microbiol. Biotechnol. 34 (1991) 105–116
Hammerberg, B.; Nagamune, T.; Endo, I.; Moks, T.; Uhlén, M.: Utilizing the tubular bioreactor for the continuous recovery of secreted fusion protein from recombinant Escherichia coli. Bioprecess Eng. 7 (1991) 145–150
Kawakatsu, T.; Miwa, T.; Matsumoto, Y.; Nakao, S.I.; Kimura, S.: Continuous production of β-galactosidase in repeated batch culture of Penicillium multicolor with a membrane enclosed reactor. J. Ferment. Bioeng. 75 (1991) 126–131
Nipkow, A.; Zeikus, J.G.; Gerhardt, P.: Microfiltration cell-recycle pilot system for continuous thermoanaerobic production of exo-β-Amylase. Biotechnol. Bioeng. 34 (1989) 1075–1084
Ponzo, J.H.; Weigand, W.A.: Simple structured model for α-amylase synthesis in Bacillus amyloliquefaciens. Biotechnol. Bioeng. 38 (1991) 1065–1081
Pasari, A.B.; Korus, R.A.; Heimsch, R.C.: A model for continuous fermentation with amolytic yeasts. Biotechnol. Bioeng. 33 (1989) 338–343
Castro, G.R.; Méndez, B.S.; Siñeriz, F.: Amylolytic enzymes produced by Bacillus amyloliquefaciens MIR-41 in batch and continuous culture. J. Chem. Technol. Biotechnol. 56 (1993) 289–294
Yamamoto, T.: Bacterial α-amylases of Bacillus subtilis and related bacteria. In: Handbook of amylases and related enzymes. The amylase research society of Japan, pp. 40–44. Pergamon Press, Oxford 1989
Mountain, A.: Gene expression systems. In: Bacillus. Harwood, C.R. (Ed.): Biotechnology Handbooks, 2. ed. pp 99–197. Plenum Press, N.Y. 1989
Emborg, C.; Jepsen, P.K.; Biedermann, L.: Two-level factorial screening of new plasmid/strain combinations for production of recombinant-DNA products. Biotechnol. Bioeng. 33 (1989) 1393–1399
Jepsen, P.K.; Riise, E.; Biedermann, K.; Kristensen, P.C.R.; Emborg, C.: Two-level factorial screening for influence of temperature, pH, and aeration on production of Serratia marcesens, nuclease. Appl. Environ. Microbiol. 53 (1987) 2593–2596
Biedermann, K.; Fiedler, H.; Larsen, B.S.; Riise, E.; Emborg, C.; Jepsen, P.K.: Fermentation studies of the secretion of Serratia marcesens nuclease by Escherichia coli. Appl. Environ. Microbiol. 56 (1990) 1833–1838
Mortensen, U.; Noorman H.J. (eds.): Proceedings on bioreactor performance 15.–17. March 1993, Helsingør, Denmark, The Biotechnology Research Foundation, Lund, Sweden1993)
Mortensen, U.; Luyben, K.C.A.M.; Moser, A. (eds.): Nordic Programme on Bioprocess Engineering 1989–1993, Scientific Report. The Biotechnology Research Foundation, Lund, Sweden (1993)
Toda, K.; Takeuchi, T.; Sano, H.: Growth rate dependence of enzyme synthesis in chemostat cultures: α-amylase, β-galactosidase, acid phosphatase and β-fructosidase. J. Chem. Technol. Biotechnol. 29 (1979) 747–755
Heineken, F.G.; O'Connor, R.J.: Continuous culture studies on the biosynthesis of alkaline protease, neutral protease and α-amylase by Bacillus subtilis NRRL-B3411. J. Gen. Microbiol. 73 (1972) 35–44
Bull, D.N.; Young, M.D.: Enhanced product formation in continuous fermentations with microbial cell recycle. Biotechnol. Bioeng. 23 (1981) 373–389
Karl, D.W.; Donovan, M.; Flickninger, M.C.: A novel acid proteinase released by hybridoma cells. Cytotechnology 3 (1990) 157–169
Mitsuda S.; Matsuda Y.; Kobayashi N.; Suzuki; Itagaki Y.; Kumazawa K.; Kawanishi G.: Tissue plasminogen activator (t-PA) production by human fibroblasts using a bioreactor with (t-PA) production by human fibroblasts using a bioreactor with t-PA adsorption column. Bioprocess Eng. 7 (1991) 137–140
Olsvik, E.S.; Kristianson B.: Influence of oxygen tension, cell mass concentration, and specific growth rate on the rheological properties of a filamentous fermentation broth. Biotech. Bioeng. 40 (1992) 1293–1299
Author information
Authors and Affiliations
Additional information
This study was supported by the Nordic Industrial Foundation Bioprocess Engineering Programme and the Center for Process Biotechnology, The Technical University of Denmark.
Rights and permissions
About this article
Cite this article
Grøn, S., Emborg, C. & Biedermann, K. Enzyme production in a cell recycle fermentation system evaluated by computer simulations. Bioprocess Engineering 13, 59–68 (1995). https://doi.org/10.1007/BF00420431
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00420431