Abstract
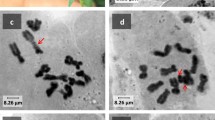
The centromeric region of a telocentric field bean chromosome that resulted from centric fission of the metacentric satellite chromosome was microdissected. The DNA of this region was amplified and biotinylated by degenerate oligonucleotide-primed polymerase chain reaction (DOP-PCR)/linker-adapter PCR. After fluorescence in situ hybridization (FISH) the entire chromosome complement of Vicia faba was labelled by these probes except for the nucleolus organizing region (NOR) and the interstitial heterochromatin, the chromosomes of V. sativa and V. narbonensis were only slightly labelled by the same probes. Dense uniform labelling was also observed when a probe amplified from a clearly delimited microdissected centromeric region of a mutant of Tradescantia paludosa was hybridized to T. paludosa chromosomes. Even after six cycles of subtractive hybridization between DNA fragments amplified from centromeric and acentric regions no sequences specifically located at the field bean centromeres were found among the remaining DNA. A mouse antiserum was produced which detected nuclear proteins of 33 kDa and 68 kDa; these were predominantly located at V. faba kinetochores during mitotic metaphase. DNA amplified from the chromatin fraction adsorbed by this serum out of the sonicated total mitotic chromatin also did not cause specific labelling of primary constrictions. From these results we conclude: (1) either centromere-specific DNA sequences are not very conserved among higher plants and are — at least in species with large genomes — intermingled with complex dispersed repetitive sequences that prevent the purification of the former, or (2) (some of) the dispersed repeats themselves specify the primary constrictions by stereophysical parameters rather than by their base sequence.
Similar content being viewed by others
References
Alfenito MR, Birchler JA (1993) Molecular characterization of a maize B chromosome centric sequence. Genetics 135:589–597
Archidiacono N, Antonacci R, Marzella R, Finelli P, Lonoce A, Rocchi M (1995) Comparative mapping of human alphoid sequences in great apes using fluorescence situ hybridization. Genomics 25:477–484
Binarova P, Cihalikova J, Dolezel J (1993) Localization of MPM-2 recognized phosphoproteins and tubulin during cell cycle progression in synchronized Vicia faba root meristem cells. Cell Biol Int 17:847–856
Brenner S, Peper D, Berns MW, Tan E, Brinkley BR (1981) Kinetochore structure, duplication, and distribution in mammalian cells: analysis by human autoantibodies from scleroderma patients. J Cell Biol 91:95–102
Clarke L, Baum M, Marschall LG, Ngan VK, Steiner NC (1993) Structure and function of Schizosaccharomyces pombe centromeres. Cold Spring Harbor Symp Quant Biol 58:687–695
Döbel P, Schubert I, Rieger R (1978) Distribution of heterochromatin in a reconstructed karyotype of Vicia faba as identified by banding- and DNA-late replication patterns. Chromosoma 69:193–209
Earnshaw WC (1991) When is a centromere not a kinetochore? J Cell Sci 99:1–4
Earnshaw WC, MacKay AM (1994) Role of nonhistone proteins in the chromosomal events of mitosis. FASEB J 8:947–956
Earnshaw WC, Rothfield N (1985) Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma 91:313–321
Fuchs J, Schubert I (1995) Localization of seed protein genes on metaphase chromosomes of Vicia faba via fluorescence situ hybridization. Chromosome Res 3:94–100
Fuchs J, Pich U, Meister A, Schubert I (1994) Differentiation of field bean heterochromatin by in situ hybridization with a repeated FokI sequence. Chromosome Res 2:25–28
Fuchs J, Houben A, Brandes A, Schubert I (1996) Chromosome ‘painting’ in plants — a feasible technique? Chromosoma 104:315–320
Haaf T, Warburton PE, Willard HF (1992) Integration of human α-satellite DNA into simian chromosomes: centromere protein binding and disruption of normal chromosome segregation. Cell 70:681–696
Hadlaczky G, Went M, Ringertz NR (1986) Direct evidence for the non-random localization of mammalian chromosomes in the interphase nucleus. Exp Cell Res 167:1–15
Hadlaczky G, Praznovszky T, Cserpán I, Keresö J, Péterfy M, Kelemen I, Atalay E, Szeles A, Szelei J, Tubak V, Burg K (1991) Centromere formation in mouse cells cotransformed with human DNA and a dominant marker gene. Proc Natl Acad Sci USA 88:8106–8110
Harrison GE, Heslop-Harrison JS (1995) Centromeric repetitive DNA sequences in the genus Brassica. Theor Appl Genet 90:157–165
Heus JJ, Bloom KS, Zonneveld BJM, Steensma HY, Van den Berg JA (1993) Chromatin structures of Kluyveromyces lactis centromeres in K. lactis and Saccharomyces cerevisiae. Chromosoma 102:660–667
Houben A, Guttenbach M, Kreß W, Pich U, Schubert I, Schmid M (1995) Immunostaining and interphase arrangement of field bean kinetochores. Chromosome Res 3:27–31
Houben A, Franke J, Leclerc N, Ahne R (1996) A computer assisted system combining image analysis and chromosome dissection. Microsc Res Technol (in press)
Jiang J, Gill BS (1994) Non-isotopic in situ hybridization and plant genome mapping: the first 10 years. Genome 37:717–725
Johnson DH (1990) Molecular cloning of DNA from specific chromosomal regions by microdissection and sequence-independent amplification of DNA. Genomics 6:243–251
Leach CR, Donald TM, Franks TK, Spiniello SS, Hanrahan CF, Timmis JN (1995) Organization and origin of a B chromosome centromeric sequence from Brachycome dichromosomatica. Chromosoma 103:708–714
Liao H, Winkfein RJ, Mack G, Rattner JB, Yen TJ (1995) CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J Cell Biol 130:507–518
Loidl P (1988) Towards an understanding of the biological function of histone acetylation. FEBS Lett 227:91–95
Maluszynska J, Heslop-Harrison JS (1991) Localization of tandemly repeated DNA sequences in Arabidopsis thaliana. Plant J 1:159–166
Mole-Bajer J, Bajer AS, Zinkowski RP, Balczon RD, Brinkley BR (1990) Autoantibodies from a patient with scleroderma CREST recognized kinetochores of the higher plant Haemanthus. Proc Natl Acad Sci USA 87:3599–3603
Moroi Y, Peebles C, Fritzler MJ, Steigerwald J, Tan EM (1980) Autoantibody to centromere (kinetochore) in scleroderma sera. Proc Natl Acad Sci USA 77:1627–1631
Murata M, Ogura Y, Motoyoshi F (1994) Centromeric repetitive sequences in Arabidopsis thaliana. Jpn J Genet 69:361–370
Murphy TD, Karpen GH (1995) Localization of centromere function in a Drosophila minichromosome. Cell 82:599–609
Ouspenski II, Brinkley BR (1993) Centromeric DNA cloned from functional kinetochore fragments in mitotic cells with unreplicated genomes. J Cell Sci 105:359–367
Page SL, Earnshaw WC, Choo KHA, Shaffer LG (1995) Further evidence that CENP-C is a necessary component of active centromeres: studies of a dic(X;15) with simultaneous immunofluorescence and FISH. Hum Mol Genet 4:289–294
Palevitz BA (1990) Kinetochore behavior during generative cell division in Tradescantia virginiana. Protoplasma 157:120–127
Pich U, Houben A, Fuchs J, Meister A, Schubert I (1994) Utility of DNA amplified by degenerate oligonucleotide-primed PCR (DOP-PCR) from the total genome and defined chromosomal regions of field beans. Mol Gen Genet 243:173–177
Pluta AF, Mackay AM, Ainsztein AM, Goldberg IG, Earnshaw WC (1995) The centromere: hub of chromosomal activities. Science 270:1591–1594
Schmit A-C, Stoppin V, Chevrier V, Job D, Lambert A-M (1994) Cell cycle dependent distribution of a centrosomal antigen at the perinuclear MTOC or at the kinetochores of higher plant cells. Chromosoma 103:343–351
Schubert I, Rieger R (1990) Alteration by centric fission of the diploid chromosome number in Vicia faba L. Genetica 81:67–69
Schubert I, Rieger R (1991) Catalogue of chromosomal and morphological mutants of Faba bean in the Gatersleben Collection, 1991. FABIS Newsl 28/29:14–22
Schubert I, Dolezel J, Houben A, Scherthan H, Wanner G (1993) Refined examination of plant metaphase chromosome structure at different levels made feasible by new isolation methods. Chromosoma 102:96–101
Shaw DD (1994) Centromes: moving chromosomes through space and time. Trends Ecol Evol 9:170–175
Sullivan BA, Schwartz S (1995) Identification of centromeric antigens in dicentric Robertsonian translocations: CENP-C and CENP-E are necessary compoments of functional contromeres. Hum Mol Genet 4:2189–2197
Sunkel CE, Coelho PA (1995) The elusive centromere: sequence divergence and functional conservation. Curr Opinion Genet Dev 5:756–767
Tagle DA, Swaroop M, Lovett M, Collins FS (1993) Magnetic bead capture of expressed sequences encoded within large genomic segments. Nature 361:751–753
Telenius H, Carter NP, Bebb CE, Nordenskjöld M, Ponder BAJ, Tunnacliffe A (1992) Degenerate oligonucleotide-primed PCR: general amplification of target DNA by a single degenerate primer. Genomics 13:718–725
Turner BM (1993) Decoding the nucleosome. Cell 75:5–8
Tyler-Smith C, Oakey RJ, Larin Z, Fisher RB, Crocker M, Affara NA, Ferguson-Smith MA, Muenke M, Zuffardi O, Jobling MA (1993) Localization of DNA sequences required for human centromere function through an analysis of rearranged Y chromosomes. Nature Genet 5:368–375
van Holde KE (1988) Chromatin. Springer, Berlin Heidelberg New York
Vig BK (1994) Do specific nucleotide bases constitute the centromere? Mutat Res 309:1–10
Vig BK, Latour D, Frankovich J (1994) Dissociation of minor satellite from the centromere in mouse. J Cell Sci 107:3091–3095
Yen TJ, Compton DA, Wise D, Zinkowski RP, Brinkley BR, Earnshaw WC, Cleveland DW (1991) CENP-E, a novel human centromere-associated protein required for progression from metaphase to anaphase. EMBO J 10:1245–1254
Author information
Authors and Affiliations
Additional information
Communicated by F. Mechelke
Rights and permissions
About this article
Cite this article
Houben, A., Brandes, A., Pich, U. et al. Molecular-cytogenetic characterization of a higher plant centromere/kinetochore complex. Theoret. Appl. Genetics 93, 477–484 (1996). https://doi.org/10.1007/BF00417938
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00417938