Abstract
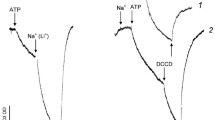
Chloride uptake by the cyanobacterium Anacystis nidulans at 38°C is energy dependent showing maximum rate (around 5.10-7 mol Cl-xml cell water-1xmin-1) and accumulation (up to 160 fold) in light and air. The respective values in air and darkness were 40–70% lower. In the dark under N2 no uptake was found. Chloride transport had an optimum at pH 6.7 and a K M of 2.10-5 M which was pH-independent. It was inhibited by carbonyl cyanide m-chlorophenylhydrazone and N,N′-dicyclohexylcarbodiimide in the light and in the dark, and also to a lesser extent by valinomycin. 3-(3,4-dichlorophenyl)-1,1-dimethylurea in the light caused a moderate stimulation.
To obtain information about the energy source of active chloride transport the action of the four inhibitors on membrane potential (determined through the distribution of triphenylmethylphosphonium) and ATP level (determined by the firefly method) was examined. It was found that a high negative membrane potential was unfavorable for chloride accumulation probably by stimulating passive efflux. On the other hand a good correlation between ATP level and chloride transport activity was obtained.
Attempts to induce chloride uptake by sudden acidification of the external medium in presence of N,N′-dicyclohexyl-carbodiimide or during anaerobiosis were not successful.
Two mechanisms of chloride uptake are discussed:
-
a)
primary active transport by an ATP-dependent pump, and
-
b)
“chemiosmotic” secondary active transport linked to a proton gradient, the present data favoring mechanism a.
Similar content being viewed by others
Abbreviations
- CCCP:
-
carbonyl cyanide m-chlorophenylhydrazone
- DA:
-
daik/air conditions
- DN:
-
daik/nitrogen conditions
- DCCD:
-
N,N-dicyclohexylcarbodiimide
- DCMU:
-
3-(3,4-dichlorophenyl)-1,1-dimethylurea
- Hepes:
-
N-2-hydroxyethylpiperazine-N′-ethanesulfonic acid
- LA:
-
light/air conditions
- TPMP+ :
-
trimethylphenylphosphonium
- Tris:
-
tris-hydroxymethylaminomethane
- PPO:
-
2,5-diphenyloxazole
- POPOP:
-
1,4-bis-2-(4-methyl-5-phenyl-oxazolyl)-benzene
References
Bakeeva LE, Grinius LL, Jasaitis AA, Kuliesse VV, Leritzky DO, Liberman EA, Severina II, Skulachev VP (1970) Conversion of biomembrane-produced energy into electric form. II. Intact mitochondria. Biochim Biophys Acta 216:13–21
Barber J (1968) Measurement of the membrane potential and evidence for active transport of ions in Chlorella pyrenoidosa. Biochim Biophys Acta 150:618–625
Bornefeld T, Simonis W (1974) Effects of light, temperature pH; and inhibitors on the ATP level of the blue-green alga Anacystis nidulans. Planta 115:309–318
Dewar MA, Barber J (1973) Cation regulation in Anacystis nidulans. Planta 113:143–155
Dewar MA, Barber J (1974) Chloride transport in Anacystis nidulans. Planta 117:163–172
Falkner G, Horner F, Werdan K, Heldt HW (1976) pH changes in the cytoplasm of the blue-green alga Anacystis nidulans caused by light dependent proton flux into the thylakoid space. Plant Physiol 58:717–718
Felle H, Bentrup FW (1977) A study of the primary effect of the uncoupler carbonyl cyanide m-chlorophenylhydrazone on membrane potential and conductance in Riccia fluitans. Biochim Biophys Acta 464:179–187
Harold FM (1977) Ion currents and physiological functions in microorganisms. Ann Rev Microbiol 31:181–203
Harold FM, Papineau D (1972) Cation transport and electrogenesis by Streptococcus faecalis. I. The membrane potential. J Membrane Biol 8:27–44
Hill AE, Hill BS (1973a) The electrogenic chloride pump of the Limonium salt gland. J Membr Biol 12:129–144
Hill AE, Hill BS (1973b) ATP-driven chloride pumping and ATPase activity in the Limonium salt gland. J Membr Biol 12:145–158
Kratz MA, Myers J (1955) Nutrition and growth of several blue-green algae. Amer J Bot 42:282–287
Larsson CM, Olsson T (1979) Firefly assay of adenine nucleotides from algae: Comparison of extraction methods. Plant and Cell Physiol 20:145–155
MacRobbie EAC (1965) The nature of the coupling between light energy and activeion transport in Nitella translucens. Biochim Biophys Acta 94:64–73
MacRobbie EAC (1975) Ion transport in plant cells. In: Bronner F, Kleinzeller A (eds) Current topics in membranes and transport. Academic Press, New York San Francisco London, pp 1–48
Paschinger H (1977) DCCD-induced sodium uptake by Anacystis nidulans. Arch Microbiol 113:285–291
Poole RJ (1978) Energy coupling for membrane transport. Ann Rev Plant Physiol 29:437–460
Raven JA (1967) Light stimulation of active transport in Hydrodictyon africanum. J Gen Physiol 50:1627–1640
Rottenberg H (1976) The driving force for proton(s) metabolites cotransport in bacterial cells. FEBS Lett 66:159–163
Schuldiner S, Kaback HR (1975) Membrane potential and active transport in membrane vesicles from E. coli. Biochemistry 14:5451–5461
Simonis W, Bornefeld T, Lee-Kaden J, Majumdar K (1974) Phosphate uptake and photophosphorylation in the blue-green alga Anacystis nidulans. In: Zimmermann U, Dainty J (eds) Membrane transport in plants. Springer, Berlin Heidelberg New York, pp 220–225
Smith FA, Walker NA (1976) Chloride transport in Chara corallina and the electrochemical potential difference for hydrogen, ions. J Exp Bot 27:451–459
Walker NA, Smith FA (1975) Intracellular pH in Chara corallina measured by DMO distribution. Plant Sci Lett 4:125–132
Wittig G, Schöllkopf U (1954) Über Triphenylphosphinmethylene als olefinbildende Reagenzien. Chem Ber 87:1318–1330
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zdrou, I., Tromballa, H.W. Active transport of chloride by Anacystis nidulans . Arch. Microbiol. 129, 325–330 (1981). https://doi.org/10.1007/BF00414707
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00414707