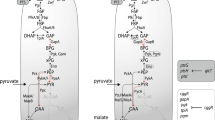
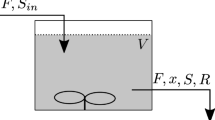
Abstract
Aerobic growth of Escherichia coli and Paracoccus denitrificans has been studied in chemostat, fed batch, and recycling fermentor modes under carbon and energy limitation. Two abrupt drops or discontinuities in molar growth yield, Y, have been found that occur over relatively short ranges in the value of specific growth rate.
Before the first discontinuity, Y is constant and maximal. After the first discontinuity, at a doubling time of 33 h, Y becomes constant again and independent of μ until the second discontinuity appears at a doubling time of about 50 h, corresponding to a μ of about 0.014. At this point, Y drops to a lower value that is constant at doubling times longer than 100 h, corresponding to a μ of about 0.007.
The second discontinuity is associated in Paracoccus with elevated levels of guanosine tetraphosphate (ppGpp) that impose stringent regulation as has been found previously with Bacillus and Escherichia species. It is thus likely that the stringent response generally occurs in bacteria in vivo at a doubling time of about 50 h. The cause of the first discontinuity is unknown. All experiments indicate that Pirt-type calculations relating μ, Y, and maintenance energy demand are no longer valid. In chemostat experiments, the intercept of the relationship between specific substrate utilization and specific growth rate is defined as maintenance. However, this intercept most probably is caused by stringent regulation at low dilution rates. Three regions of bacterial growth rates are defined by this study, corresponding to doubling times of 0.5 to 15 h, 33 to 50 h, and >100 h. Some growth behavior in each region is unique to that region.
Similar content being viewed by others
Abbreviations
- ppGpp:
-
guanosine 5′ diP 3′ diP
- pppGpp:
-
guanosine 5′ triP 3′ diP
- SPR:
-
substrate provision rate (mol/l h)
References
Arbige M, Chesbro WR (1982a) Rel A and related loci are growth rate determinants for Escherichia coli in a recycling fermentor. J Gen Microbiology 128:693–703
Arbige M, Chesbro WR (1982b) Very slow growth of Bacillus polymyxa: stringent response and maintenance energy. Arch Microbiol 132:338–344
Chesbro WR, Evans T, Eifert R (1979) Very slow growth of Escherichia coli. J Bacteriol 139:625–638
Fox GE, Stackebrandt E, Hespell RB, Gibson J, Manilof J, Dyer TA, Wolfe RS, Balch WE, Tanner RS, Magrum LJ, Zablen LB, Blakemore RP, Gupta R, Bonen L, Lewis BJ, Stahl DA, Leuhrsen KR, Chen KN, Woese CR (1980) The phylogeny of prokaryotes. Science 209:457–463
Gallant JA (1979) Stringent control in Escherichia coli. Ann Rev Gen 13:393–415
Gallant JA, Foley D (1979) Stringent control of transitional accuracy. In: Koch G, Richter D (eds) Regulation of macromolecular synthesis by low molecular weight mediators. Acad Press, New York, pp 5–24
Gallant JA, Harada B (1969) The control of ribonucleic acid synthesis in Escherichia coli. III. The functional relationship between purine ribonucleoside triphosphate pool sizes and the rate of ribonucleic acid accumulation. J Biol Chem 244:3125–3132
Lagosky P, Chang FN (1980) Influence of amino acid starvation on ppGpp basal level synthesis in Escherichia coli. J Bacteriol 144:499–508
Light PA, Garland PB (1971) A comparison of mitochondria from Torulopsis utilis grown in continuous culture with glycerol. Iron, ammonium, magnesium or phosphate as the growth limiting nutrient. Biochem J 124:123–134
Neijssel OM, Tempest DW (1976) Bioenergetic aspects of aerobic growth of Klebsiella aerogenes NCTC 418 in carbon-limited and carbon-sufficient chemostat culture. Arch Microbiol 107:215–221
Pirt SJ (1965) The maintenance energy of bacteria in growing cultures. Proc Roy Soc B 163:224–231
Pirt SJ (1982) Maintenance energy: a general model for energylimited and energy-sufficient growth. Arch Microbiol 133:300–302
Stouthamer AH, Bettenhaussen CW (1975) Determination of the efficiency of oxidative phosphorylation in continous cultures of Aerobacter aerogenes. Arch Microbiol 102:187–192
Verseveld HW van (1979) Influence of environmental factors on the efficiency of energy conservation in Paracoccus denitrificans. Ph. D. thesis, Vrije Universiteit, Amsterdam
Verseveld HW van, Boon JP, Stouthamer AH (1979) Growth yields and the efficiency of oxidative phosphorylation of Paracoccus denitrificans during two-(carbon) substrate-limited growth. Arch Microbiol 121:213–223
Verseveld HW van, Stouthamer AH (1976) Oxidative phosphorylation in Micrococcus denitrificans. Calculation of the P/O ratio in growing cells. Arch Microbiol 107:241–247
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Verseveld, H.W., Chesbro, W.R., Braster, M. et al. Eubacteria have 3 growth modes keyed to nutrient flow. Arch Microbiol 137, 176–184 (1984). https://doi.org/10.1007/BF00414463
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00414463